Expanding the Phenotype Associated with NAA10-Related N-Terminal Acetylation Deficiency
- PMID: 27094817
- PMCID: PMC5084832
- DOI: 10.1002/humu.23001
Expanding the Phenotype Associated with NAA10-Related N-Terminal Acetylation Deficiency
Abstract
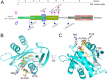
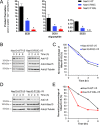
N-terminal acetylation is a common protein modification in eukaryotes associated with numerous cellular processes. Inherited mutations in NAA10, encoding the catalytic subunit of the major N-terminal acetylation complex NatA have been associated with diverse, syndromic X-linked recessive disorders, whereas de novo missense mutations have been reported in one male and one female individual with severe intellectual disability but otherwise unspecific phenotypes. Thus, the full genetic and clinical spectrum of NAA10 deficiency is yet to be delineated. We identified three different novel and one known missense mutation in NAA10, de novo in 11 females, and due to maternal germ line mosaicism in another girl and her more severely affected and deceased brother. In vitro enzymatic assays for the novel, recurrent mutations p.(Arg83Cys) and p.(Phe128Leu) revealed reduced catalytic activity. X-inactivation was random in five females. The core phenotype of X-linked NAA10-related N-terminal-acetyltransferase deficiency in both males and females includes developmental delay, severe intellectual disability, postnatal growth failure with severe microcephaly, and skeletal or cardiac anomalies. Genotype-phenotype correlations within and between both genders are complex and may include various factors such as location and nature of mutations, enzymatic stability and activity, and X-inactivation in females.
Keywords: N-terminal acetylation; NAA10; X-linked; intellectual disability.
© 2016 The Authors. **Human Mutation published by Wiley Periodicals, Inc.
Figures

References
-
- Aksnes H, Hole K, Arnesen T. 2015. Molecular, cellular, and physiological significance of N‐terminal acetylation. Int Rev Cell Mol Biol 316:267–305. - PubMed
-
- Arnesen T, Van Damme P, Polevoda B, Helsens K, Evjenth R, Colaert N, Varhaug JE, Vandekerckhove J, Lillehaug JR, Sherman F, Gevaert K. 2009. Proteomics analyses reveal the evolutionary conservation and divergence of N‐terminal acetyltransferases from yeast and humans. Proc Natl Acad Sci U S A 106:8157–8162. - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

