Mutations in human C2CD3 cause skeletal dysplasia and provide new insights into phenotypic and cellular consequences of altered C2CD3 function
- PMID: 27094867
- PMCID: PMC4837335
- DOI: 10.1038/srep24083
Mutations in human C2CD3 cause skeletal dysplasia and provide new insights into phenotypic and cellular consequences of altered C2CD3 function
Abstract
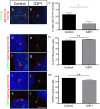
Ciliopathies are a group of genetic disorders caused by defective assembly or dysfunction of the primary cilium, a microtubule-based cellular organelle that plays a key role in developmental signalling. Ciliopathies are clinically grouped in a large number of overlapping disorders, including the orofaciodigital syndromes (OFDS), the short rib polydactyly syndromes and Jeune asphyxiating thoracic dystrophy. Recently, mutations in the gene encoding the centriolar protein C2CD3 have been described in two families with a new sub-type of OFDS (OFD14), with microcephaly and cerebral malformations. Here we describe a third family with novel compound heterozygous C2CD3 mutations in two fetuses with a different clinical presentation, dominated by skeletal dysplasia with no microcephaly. Analysis of fibroblast cultures derived from one of these fetuses revealed a reduced ability to form cilia, consistent with previous studies in C2cd3-mutant mouse and chicken cells. More detailed analyses support a role for C2CD3 in basal body maturation; but in contrast to previous mouse studies the normal recruitment of the distal appendage protein CEP164 suggests that this protein is not sufficient for efficient basal body maturation and subsequent axonemal extension in a C2CD3-defective background.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

