Chromatin folding and DNA replication inhibition mediated by a highly antitumor-active tetrazolato-bridged dinuclear platinum(II) complex
- PMID: 27094881
- PMCID: PMC4837362
- DOI: 10.1038/srep24712
Chromatin folding and DNA replication inhibition mediated by a highly antitumor-active tetrazolato-bridged dinuclear platinum(II) complex
Abstract
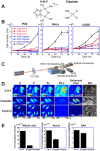
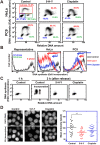
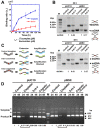
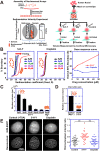
Chromatin DNA must be read out for various cellular functions, and copied for the next cell division. These processes are targets of many anticancer agents. Platinum-based drugs, such as cisplatin, have been used extensively in cancer chemotherapy. The drug-DNA interaction causes DNA crosslinks and subsequent cytotoxicity. Recently, it was reported that an azolato-bridged dinuclear platinum(II) complex, 5-H-Y, exhibits a different anticancer spectrum from cisplatin. Here, using an interdisciplinary approach, we reveal that the cytotoxic mechanism of 5-H-Y is distinct from that of cisplatin. 5-H-Y inhibits DNA replication and also RNA transcription, arresting cells in the S/G2 phase, and are effective against cisplatin-resistant cancer cells. Moreover, it causes much less DNA crosslinking than cisplatin, and induces chromatin folding. 5-H-Y will expand the clinical applications for the treatment of chemotherapy-insensitive cancers.
Figures


References
-
- Watson J. D. et al. Molecular Biology of the Gene, 7/E. (Benjamin Cummings, 2013).
-
- Davey G. E. & Davey C. A. Chromatin - a new, old drug target? Chem Biol Drug Des 72, 165–170 (2008). - PubMed
-
- Rosenberg B. & VanCamp L. The successful regression of large solid sarcoma 180 tumors by platinum compounds. Cancer Res 30, 1799–1802 (1970). - PubMed
-
- Kociba R. J., Sleight S. D. & Rosenberg B. Inhibition of Dunning asc itic leukemia and Walker 256 carcinosarcoma with cis-diamminedichloroplatinum (NSC-119875). Cancer Chemother Rep 54, 325–328 (1970). - PubMed
-
- Mansy S., Rosenberg B. & Thomson A. J. Binding of cis- and trans-dichlorodiammineplatinum(II) to nucleosides. I. Location of the binding sites. J Am Chem Soc 95, 1633–1640 (1973). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

