Personalized fludarabine dosing to reduce nonrelapse mortality in hematopoietic stem-cell transplant recipients receiving reduced intensity conditioning
- PMID: 27094990
- PMCID: PMC5003687
- DOI: 10.1016/j.trsl.2016.03.017
Personalized fludarabine dosing to reduce nonrelapse mortality in hematopoietic stem-cell transplant recipients receiving reduced intensity conditioning
Abstract
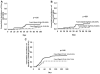
Patients undergoing hematopoietic cell transplantation (HCT) with reduced intensity conditioning (RIC) commonly receive fludarabine. Higher exposure of F-ara-A, the active component of fludarabine, has been associated with a greater risk of nonrelapse mortality (NRM). We sought to develop a model for fludarabine dosing in adult HCT recipients that would allow for precise dose targeting and predict adverse clinical outcomes. We developed a pharmacokinetic model from 87 adults undergoing allogeneic RIC HCT that predicts F-ara-A population clearance (Clpop) accounting for ideal body weight and renal function. We then applied the developed model to an independent cohort of 240 patients to identify whether model predictions were associated with NRM and acute graft versus host disease (GVHD). Renal mechanisms accounted for 35.6% of total F-ara-A Clpop. In the independent cohort, the hazard ratio of NRM at day 100 was significantly higher in patients with predicted F-ara-A clearance (Clpred) <8.50 L/h (P < 0.01) and area under the curve (AUCpred) >6.00 μg × h/mL (P = 0.01). A lower Clpred was also associated with more NRM at month 6 (P = 0.01) and trended toward significance at 12 months (P = 0.05). In multivariate analysis, low fludarabine clearance trended toward higher risk of acute GVHD (P = 0.05). We developed a model that predicts an individual's systemic F-ara-A exposure accounting for kidney function and weight. This model may provide guidance in dosing especially in overweight individuals and those with altered kidney function.
Copyright © 2016 Elsevier Inc. All rights reserved.
Conflict of interest statement
All authors have read the journal's authorship agreement and policy on disclosure of conflict of interest. None of the authors have any conflicts of interest and all authors approved this publication.
Figures
Similar articles
-
Association of fludarabine pharmacokinetic/dynamic biomarkers with donor chimerism in nonmyeloablative HCT recipients.Cancer Chemother Pharmacol. 2015 Jul;76(1):85-96. doi: 10.1007/s00280-015-2768-x. Epub 2015 May 17. Cancer Chemother Pharmacol. 2015. PMID: 25983023 Free PMC article.
-
Pharmacokinetics and Model-Based Dosing to Optimize Fludarabine Therapy in Pediatric Hematopoietic Cell Transplant Recipients.Biol Blood Marrow Transplant. 2017 Oct;23(10):1701-1713. doi: 10.1016/j.bbmt.2017.06.021. Epub 2017 Jul 3. Biol Blood Marrow Transplant. 2017. PMID: 28684371 Free PMC article. Clinical Trial.
-
Fludarabine exposure in the conditioning prior to allogeneic hematopoietic cell transplantation predicts outcomes.Blood Adv. 2019 Jul 23;3(14):2179-2187. doi: 10.1182/bloodadvances.2018029421. Blood Adv. 2019. PMID: 31324638 Free PMC article.
-
Fludarabine/Busulfan versus Fludarabine/Melphalan Conditioning in Patients Undergoing Reduced-Intensity Conditioning Hematopoietic Stem Cell Transplantation for Lymphoma.Biol Blood Marrow Transplant. 2016 Oct;22(10):1808-1815. doi: 10.1016/j.bbmt.2016.07.006. Epub 2016 Jul 25. Biol Blood Marrow Transplant. 2016. PMID: 27470290
-
Treatment-related mortality and graft-versus-leukemia activity after allogeneic stem cell transplantation for chronic lymphocytic leukemia using intensity-reduced conditioning.Leukemia. 2003 May;17(5):841-8. doi: 10.1038/sj.leu.2402905. Leukemia. 2003. PMID: 12750695 Review.
Cited by
-
A hybrid machine learning/pharmacokinetic approach outperforms maximum a posteriori Bayesian estimation by selectively flattening model priors.CPT Pharmacometrics Syst Pharmacol. 2021 Oct;10(10):1150-1160. doi: 10.1002/psp4.12684. Epub 2021 Jul 26. CPT Pharmacometrics Syst Pharmacol. 2021. PMID: 34270885 Free PMC article.
-
Autologous engineered T cell receptor therapy in advanced cancer.Hum Vaccin Immunother. 2023 Dec 15;19(3):2290356. doi: 10.1080/21645515.2023.2290356. Epub 2023 Dec 19. Hum Vaccin Immunother. 2023. PMID: 38114231 Free PMC article. Review.
-
Is Monitoring of the Intracellular Active Metabolite Levels of Nucleobase and Nucleoside Analogs Ready for Precision Medicine Applications?Eur J Drug Metab Pharmacokinet. 2022 Nov;47(6):761-775. doi: 10.1007/s13318-022-00786-5. Epub 2022 Aug 1. Eur J Drug Metab Pharmacokinet. 2022. PMID: 35915365 Review.
-
The impact of age and renal function on the pharmacokinetics and protein binding characteristics of fludarabine in paediatric and adult patients undergoing allogeneic haematopoietic stem cell transplantation conditioning.Eur J Clin Pharmacol. 2024 Dec;80(12):1967-1987. doi: 10.1007/s00228-024-03751-0. Epub 2024 Sep 19. Eur J Clin Pharmacol. 2024. PMID: 39298000 Free PMC article.
-
Feasibility and Safety of Personalized, Multi-Target, Adoptive Cell Therapy (IMA101): First-in-Human Clinical Trial in Patients with Advanced Metastatic Cancer.Cancer Immunol Res. 2023 Jul 5;11(7):925-945. doi: 10.1158/2326-6066.CIR-22-0444. Cancer Immunol Res. 2023. PMID: 37172100 Free PMC article. Clinical Trial.
References
-
- Abdul Wahid SF, Ismail NA, Mohd-Idris MR, et al. Comparison of reduced-intensity and myeloablative conditioning regimens for allogeneic hematopoietic stem cell transplantation in patients with acute myeloid leukemia and acute lymphoblastic leukemia: a meta-analysis. Stem Cells and Development. 2014;23(21):2535–52. - PMC - PubMed
-
- Aoki J, Kanamori H, Tanaka M, et al. Impact of age on outcomes of allogeneic hematopoietic stem cell transplantation with reduced intensity conditioning in elderly patients with acute myeloid leukemia. Am J Hematol. 2015 Epub ahead of print. - PubMed
-
- Schneidawind D, Federmann B, Buechele C, et al. Reduced-intensity conditioning with fludarabine and busulfan for allogeneic hematopoietic cell transplantation in elderly or infirm patients with advanced myeloid malignancies. Ann Hematol. 2016;95(1):115–24. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

