Inherited platelet disorders: toward DNA-based diagnosis
- PMID: 27095789
- PMCID: PMC4972611
- DOI: 10.1182/blood-2016-03-378588
Inherited platelet disorders: toward DNA-based diagnosis
Abstract
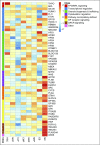
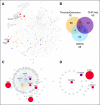
Variations in platelet number, volume, and function are largely genetically controlled, and many loci associated with platelet traits have been identified by genome-wide association studies (GWASs).(1) The genome also contains a large number of rare variants, of which a tiny fraction underlies the inherited diseases of humans. Research over the last 3 decades has led to the discovery of 51 genes harboring variants responsible for inherited platelet disorders (IPDs). However, the majority of patients with an IPD still do not receive a molecular diagnosis. Alongside the scientific interest, molecular or genetic diagnosis is important for patients. There is increasing recognition that a number of IPDs are associated with severe pathologies, including an increased risk of malignancy, and a definitive diagnosis can inform prognosis and care. In this review, we give an overview of these disorders grouped according to their effect on platelet biology and their clinical characteristics. We also discuss the challenge of identifying candidate genes and causal variants therein, how IPDs have been historically diagnosed, and how this is changing with the introduction of high-throughput sequencing. Finally, we describe how integration of large genomic, epigenomic, and phenotypic datasets, including whole genome sequencing data, GWASs, epigenomic profiling, protein-protein interaction networks, and standardized clinical phenotype coding, will drive the discovery of novel mechanisms of disease in the near future to improve patient diagnosis and management.
© 2016 by The American Society of Hematology.
Figures


References
-
- Dasouki M, Roberts J, Santiago A, Saadi I, Hovanes K. Confirmation and further delineation of the 3q26.33-3q27.2 microdeletion syndrome. Eur J Med Genet. 2014;57(2-3):76–80. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

