The Retina and Other Light-sensitive Ocular Clocks
- PMID: 27095816
- PMCID: PMC5479307
- DOI: 10.1177/0748730416642657
The Retina and Other Light-sensitive Ocular Clocks
Abstract
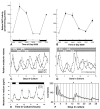
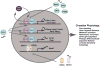
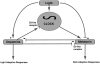
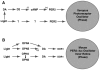
Ocular clocks, first identified in the retina, are also found in the retinal pigment epithelium (RPE), cornea, and ciliary body. The retina is a complex tissue of many cell types and considerable effort has gone into determining which cell types exhibit clock properties. Current data suggest that photoreceptors as well as inner retinal neurons exhibit clock properties with photoreceptors dominating in nonmammalian vertebrates and inner retinal neurons dominating in mice. However, these differences may in part reflect the choice of circadian output, and it is likely that clock properties are widely dispersed among many retinal cell types. The phase of the retinal clock can be set directly by light. In nonmammalian vertebrates, direct light sensitivity is commonplace among body clocks, but in mice only the retina and cornea retain direct light-dependent phase regulation. This distinguishes the retina and possibly other ocular clocks from peripheral oscillators whose phase depends on the pace-making properties of the hypothalamic central brain clock, the suprachiasmatic nuclei (SCN). However, in mice, retinal circadian oscillations dampen quickly in isolation due to weak coupling of its individual cell-autonomous oscillators, and there is no evidence that retinal clocks are directly controlled through input from other oscillators. Retinal circadian regulation in both mammals and nonmammalian vertebrates uses melatonin and dopamine as dark- and light-adaptive neuromodulators, respectively, and light can regulate circadian phase indirectly through dopamine signaling. The melatonin/dopamine system appears to have evolved among nonmammalian vertebrates and retained with modification in mammals. Circadian clocks in the eye are critical for optimum visual function where they play a role fine tuning visual sensitivity, and their disruption can affect diseases such as glaucoma or retinal degeneration syndromes.
Keywords: amacrine cell; clock; cone; dopamine; entrainment; ipRGC; melatonin; molecular clock; retina; rod.
© 2016 The Author(s).
Figures
References
-
- Adachi A, Nogi T, Ebihara S. Phase-relationship and mutual effects between circadian rhythms of ocular melatonin and dopamine in the pigeon. Brain Res. 1998;792:361–9. - PubMed
-
- Ait-Hmyed O, Felder-Schmittbuhl MP, Garcia-Garrido M, Beck S, Seide C, Sothilingam V, Tanimoto N, Seeliger M, Bennis M, Hicks D. Mice lacking Period 1 and Period 2 circadian clock genes exhibit blue cone photoreceptor defects. Eur J Neurosci. 2013;37:1048–60. - PubMed
-
- Ali MA. Retinomotor responses. In: Ali MA, editor. Vision in Fishes New Approaches in Research. N.Y.: Plenum Press; 1975. pp. 313–355.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

