Inhibition of Gαs/cAMP Signaling Decreases TCR-Stimulated IL-2 transcription in CD4(+) T Helper Cells
- PMID: 27096000
- PMCID: PMC4831273
- DOI: 10.5334/1750-2187-10-2
Inhibition of Gαs/cAMP Signaling Decreases TCR-Stimulated IL-2 transcription in CD4(+) T Helper Cells
Abstract
Background: The role of cAMP in regulating T cell activation and function has been controversial. cAMP is generally known as an immunosuppressant, but it is also required for generating optimal immune responses. As the effect of cAMP is likely to depend on its cellular context, the current study investigated whether the mechanism of activation of Gαs and adenylyl cyclase influences their effect on T cell receptor (TCR)-stimulated interleukin-2 (IL-2) mRNA levels.
Methods: The effect of blocking Gs-coupled receptor (GsPCR)-mediated Gs activation on TCR-stimulated IL-2 mRNA levels in CD4(+) T cells was compared with that of knocking down Gαs expression or inhibiting adenylyl cyclase activity. The effect of knocking down Gαs expression on TCR-stimulated cAMP accumulation was compared with that of blocking GsPCR signaling.
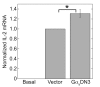
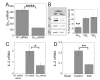
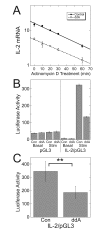
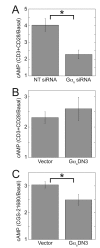
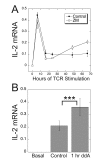
Results: ZM-241385, an antagonist to the Gs-coupled A2A adenosine receptor (A2AR), enhanced TCR-stimulated IL-2 mRNA levels in primary human CD4(+) T helper cells and in Jurkat T cells. A dominant negative Gαs construct, GαsDN3, also enhanced TCR-stimulated IL-2 mRNA levels. Similar to GsPCR antagonists, GαsDN3 blocked GsPCR-dependent activation of both Gαs and Gβγ. In contrast, Gαs siRNA and 2',5'-dideoxyadenosine (ddA), an adenylyl cyclase inhibitor, decreased TCR-stimulated IL-2 mRNA levels. Gαs siRNA, but not GαsDN3, decreased TCR-stimulated cAMP synthesis. Potentiation of IL-2 mRNA levels by ZM-241385 required at least two days of TCR stimulation, and addition of ddA after three days of TCR stimulation enhanced IL-2 mRNA levels.
Conclusions: GsPCRs play an inhibitory role in the regulation of TCR-stimulated IL-2 mRNA levels whereas Gαs and cAMP can play a stimulatory one. Additionally, TCR-dependent activation of Gαs does not appear to involve GsPCRs. These results suggest that the context of Gαs/cAMP activation and the stage of T cell activation and differentiation determine the effect on TCR-stimulated IL-2 mRNA levels.
Keywords: G-protein-coupled receptor; Gαs; IL-2; T helper cells; cAMP; heterotrimeric G-protein.
Figures

References
-
- Dumont JE, Jauniaux JC, Roger PP. The cyclic AMP-mediated stimulation of cell proliferation. Trends Biochem Sci. 1989;14(2):67–71. Epub 1989/02/01. PubMed PMID: 2539663. - PubMed
-
- Dugan LL, Kim JS, Zhang Y, Bart RD, Sun Y, Holtzman DM, et al. Differential effects of cAMP in neurons and astrocytes. Role of B-raf. J Biol Chem. 1999;274(36):25842–8. Epub 1999/08/28. PubMed PMID: 10464325. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

