Activator of G-protein Signaling 3 Controls Renal Epithelial Cell Survival and ERK5 Activation
- PMID: 27096004
- PMCID: PMC4831271
- DOI: 10.5334/1750-2187-10-5
Activator of G-protein Signaling 3 Controls Renal Epithelial Cell Survival and ERK5 Activation
Abstract
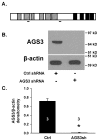
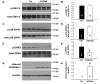
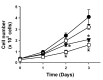
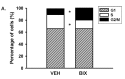
Activator of G-protein signaling 3 (AGS3) is an accessory protein that functions to regulate the activation status of heterotrimeric G-protein subunits. To date, however, the downstream signaling pathways regulated by AGS3 remain to be fully elucidated, particularly in renal epithelial cells. In the present study, normal rat kidney (NRK-52E) proximal tubular epithelial cells were genetically modified to regulate the expression of AGS3 to investigate its role on MAPK and mTOR signaling to control epithelial cell number. Knockdown of endogenous AGS3 protein was associated with a reduced phosphorylated form of ERK5 and increased apoptosis as determined by elevated cleaved caspase-3. In the presence of the ERK5 inhibitor, BIX02189, a significant 2-fold change (P < 0.05) in G2/M transition state was detected compared to control conditions. Neither of the other MAPK, ERK1/2 or p38 MAPK, nor another pro-survival pathway, mTOR, was significantly altered by the changes in AGS3 protein levels in the renal epithelial cells. The selective ERK5 inhibitor, BIX02189, was found to dose-dependently reduce NRK cell number by up to 41% (P < 0.05) compared to control cells. In summary, these findings demonstrated that cell viability was regulated by AGS3 and was associated with ERK5 activation in renal epithelial cells.
Keywords: Activator of G-protein Signaling 3; cell signaling; lentiviral vectors; renal epithelial cell.
Figures


References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

