Discovery of Clinical Development Candidate GDC-0084, a Brain Penetrant Inhibitor of PI3K and mTOR
- PMID: 27096040
- PMCID: PMC4834666
- DOI: 10.1021/acsmedchemlett.6b00005
Discovery of Clinical Development Candidate GDC-0084, a Brain Penetrant Inhibitor of PI3K and mTOR
Abstract
Inhibition of phosphoinositide 3-kinase (PI3K) signaling is an appealing approach to treat brain tumors, especially glioblastoma multiforme (GBM). We previously disclosed our successful approach to prospectively design potent and blood-brain barrier (BBB) penetrating PI3K inhibitors. The previously disclosed molecules were ultimately deemed not suitable for clinical development due to projected poor metabolic stability in humans. We, therefore, extended our studies to identify a BBB penetrating inhibitor of PI3K that was also projected to be metabolically stable in human. These efforts required identification of a distinct scaffold for PI3K inhibitors relative to our previous efforts and ultimately resulted in the identification of GDC-0084 (16). The discovery and preclinical characterization of this molecule are described within.
Keywords: CNS; PI3K; blood−brain barrier penetration; kinase inhibitor; oncology.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
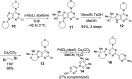
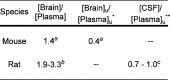



References
-
- Heffron T. P.; Salphati L.; Alicke B.; Cheong J.; Dotson J.; Edgar J.; Goldsmith R.; Gould S. E.; Lee L. B.; Lesnick J. D.; Lewis C.; Ndubaku C.; Nonomiya J.; Olivero A. G.; Pang J.; Plise E. G.; Sideris S.; Trapp S.; Wallin J.; Zhang X. The Design and Identification of Brain Penetrant Inhibitors of Phosphoinositide 3-Kinase α. J. Med. Chem. 2012, 55, 8007–8020. 10.1021/jm300867c. - DOI - PubMed
-
- Sutherlin D. P.; Bao L.; Berry M.; Castanedo G.; Chuckowree I.; Dotson J.; Folkes A.; Friedman L.; Goldsmith R.; Gunzner J.; Heffron T.; Lesnick J.; Lewis C.; Mathieu S.; Murray J.; Nonomiya J.; Pang J.; Pegg N.; Prior W. W.; Rouge L.; Salphati L.; Sampath D.; Tian Q.; Tsui V.; Wan N. C.; Wang S.; Wei B.; Wiesmann C.; Wu P.; Zhu B.-Y.; Olivero A. Discovery of a Potent, Selective, and Orally Available Class I Phosphatidylinositol 3-Kinase (PI3K)/Mammalian Target of Rapamycin (mTOR) Kinase Inhbitor (GDC-0980) for the Treatment of Cancer. J. Med. Chem. 2011, 54, 7579–7587. 10.1021/jm2009327. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

