Expansion and Activation of CD103(+) Dendritic Cell Progenitors at the Tumor Site Enhances Tumor Responses to Therapeutic PD-L1 and BRAF Inhibition
- PMID: 27096321
- PMCID: PMC4980762
- DOI: 10.1016/j.immuni.2016.03.012
Expansion and Activation of CD103(+) Dendritic Cell Progenitors at the Tumor Site Enhances Tumor Responses to Therapeutic PD-L1 and BRAF Inhibition
Abstract
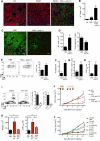
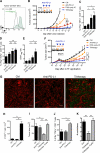
Large numbers of melanoma lesions develop resistance to targeted inhibition of mutant BRAF or fail to respond to checkpoint blockade. We explored whether modulation of intratumoral antigen-presenting cells (APCs) could increase responses to these therapies. Using mouse melanoma models, we found that CD103(+) dendritic cells (DCs) were the only APCs transporting intact antigens to the lymph nodes and priming tumor-specific CD8(+) T cells. CD103(+) DCs were required to promote anti-tumoral effects upon blockade of the checkpoint ligand PD-L1; however, PD-L1 inhibition only led to partial responses. Systemic administration of the growth factor FLT3L followed by intratumoral poly I:C injections expanded and activated CD103(+) DC progenitors in the tumor, enhancing responses to BRAF and PD-L1 blockade and protecting mice from tumor rechallenge. Thus, the paucity of activated CD103(+) DCs in tumors limits checkpoint-blockade efficacy and combined FLT3L and poly I:C therapy can enhance tumor responses to checkpoint and BRAF blockade.
Copyright © 2016 Elsevier Inc. All rights reserved.
Figures
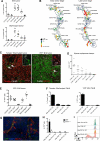
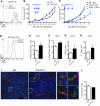
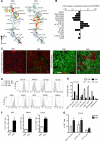
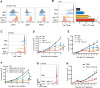
Comment in
-
Revving Up Dendritic Cells while Braking PD-L1 to Jump-Start the Cancer-Immunity Cycle Motor.Immunity. 2016 Apr 19;44(4):722-4. doi: 10.1016/j.immuni.2016.03.014. Immunity. 2016. PMID: 27096314
References
-
- Bogunovic D, O'Neill DW, Belitskaya-Levy I, Vacic V, Yu YL, Adams S, Darvishian F, Berman R, Shapiro R, Pavlick AC, et al. Immune profile and mitotic index of metastatic melanoma lesions enhance clinical staging in predicting patient survival. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:20429–20434. - PMC - PubMed
-
- Chen DS, Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity. 2013;39:1–10. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

