Scalable and sustainable electrochemical allylic C-H oxidation
- PMID: 27096371
- PMCID: PMC4860034
- DOI: 10.1038/nature17431
Scalable and sustainable electrochemical allylic C-H oxidation
Abstract
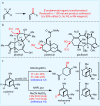
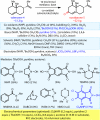
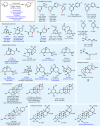
New methods and strategies for the direct functionalization of C-H bonds are beginning to reshape the field of retrosynthetic analysis, affecting the synthesis of natural products, medicines and materials. The oxidation of allylic systems has played a prominent role in this context as possibly the most widely applied C-H functionalization, owing to the utility of enones and allylic alcohols as versatile intermediates, and their prevalence in natural and unnatural materials. Allylic oxidations have featured in hundreds of syntheses, including some natural product syntheses regarded as "classics". Despite many attempts to improve the efficiency and practicality of this transformation, the majority of conditions still use highly toxic reagents (based around toxic elements such as chromium or selenium) or expensive catalysts (such as palladium or rhodium). These requirements are problematic in industrial settings; currently, no scalable and sustainable solution to allylic oxidation exists. This oxidation strategy is therefore rarely used for large-scale synthetic applications, limiting the adoption of this retrosynthetic strategy by industrial scientists. Here we describe an electrochemical C-H oxidation strategy that exhibits broad substrate scope, operational simplicity and high chemoselectivity. It uses inexpensive and readily available materials, and represents a scalable allylic C-H oxidation (demonstrated on 100 grams), enabling the adoption of this C-H oxidation strategy in large-scale industrial settings without substantial environmental impact.
Figures


References
-
- Gutekunst WR, Baran PS. C–H Functionalization logic in total synthesis. Chem. Soc. Rev. 2011;40:1976–1991. - PubMed
-
- Weidmann V, Maison W. Allylic oxidations of olefins to enones. Synthesis. 2013;45:2201–2221.
-
- Nakamura A, Nakada M. Allylic oxidations in natural product synthesis. Synthesis. 2013;45:1421–1451.
-
- Sequeira CAC, Santos DMF. Electrochemical routes for industrial synthesis. J. Braz. Chem. Soc. 2009;20:387–406.
-
- Degner D. Organic electrosyntheses in industry. In: Steckchan E, editor. Electrochemistry III. Springer; Berlin, Germany: 1988. pp. 1–95.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

