PP2A regulates kinetochore-microtubule attachment during meiosis I in oocyte
- PMID: 27096707
- PMCID: PMC4934050
- DOI: 10.1080/15384101.2016.1175256
PP2A regulates kinetochore-microtubule attachment during meiosis I in oocyte
Abstract
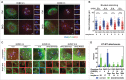
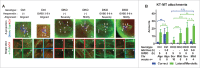
Studies using in vitro cultured oocytes have indicated that the protein phosphatase 2A (PP2A), a major serine/threonine protein phosphatase, participates in multiple steps of meiosis. Details of oocyte maturation regulation by PP2A remain unclear and an in vivo model can provide more convincing information. Here, we inactivated PP2A by mutating genes encoding for its catalytic subunits (PP2Acs) in mouse oocytes. We found that eliminating both PP2Acs caused female infertility. Oocytes lacking PP2Acs failed to complete 1(st) meiotic division due to chromosome misalignment and abnormal spindle assembly. In mitosis, PP2A counteracts Aurora kinase B/C (AurkB/C) to facilitate correct kinetochore-microtubule (KT-MT) attachment. In meiosis I in oocyte, we found that PP2Ac deficiency destabilized KT-MT attachments. Chemical inhibition of AurkB/C in PP2Ac-null oocytes partly restored the formation of lateral/merotelic KT-MT attachments but not correct KT-MT attachments. Taken together, our findings demonstrate that PP2Acs are essential for chromosome alignments and regulate the formation of correct KT-MT attachments in meiosis I in oocytes.
Keywords: KT-MT attachment; PP2A; fertility; meiosis; oocyte.
Figures




Comment in
-
PP2A in meiotic oocytes.Cell Cycle. 2016 Aug 2;15(15):1950-1. doi: 10.1080/15384101.2016.1188604. Epub 2016 May 21. Cell Cycle. 2016. PMID: 27210114 Free PMC article. No abstract available.
References
-
- Holt JE, Tran SM, Stewart JL, Minahan K, Garcia-Higuera I, Moreno S, Jones KT. The APC/C activator FZR1 coordinates the timing of meiotic resumption during prophase I arrest in mammalian oocytes. Development 2011; 138:905-13; PMID:21270054; http://dx.doi.org/10.1242/dev.059022 - DOI - PubMed
-
- Jessberger R. Age-related aneuploidy through cohesion exhaustion. EMBO Rep 2012; 13:539-46; PMID:22565322; http://dx.doi.org/10.1038/embor.2012.54 - DOI - PMC - PubMed
-
- Tachibana-Konwalski K, Godwin J, van der Weyden L, Champion L, Kudo NR, Adams DJ, Nasmyth K. Rec8-containing cohesin maintains bivalents without turnover during the growing phase of mouse oocytes. Genes Dev 2010; 24:2505-16; PMID:20971813; http://dx.doi.org/10.1101/gad.605910 - DOI - PMC - PubMed
-
- Lister LM, Kouznetsova A, Hyslop LA, Kalleas D, Pace SL, Barel JC, Nathan A, Floros V, Adelfalk C, Watanabe Y, et al.. Age-related meiotic segregation errors in mammalian oocytes are preceded by depletion of cohesin and Sgo2. Curr Biol 2010; 20:1511-21; PMID:20817533; http://dx.doi.org/10.1016/j.cub.2010.08.023 - DOI - PubMed
-
- Kitajima TS, Ohsugi M, Ellenberg J. Complete kinetochore tracking reveals error-prone homologous chromosome biorientation in mammalian oocytes. Cell 2011; 146:568-81; PMID:21854982; http://dx.doi.org/10.1016/j.cell.2011.07.031 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
