A Computational Model of the Rainbow Trout Hypothalamus-Pituitary-Ovary-Liver Axis
- PMID: 27096735
- PMCID: PMC4838294
- DOI: 10.1371/journal.pcbi.1004874
A Computational Model of the Rainbow Trout Hypothalamus-Pituitary-Ovary-Liver Axis
Abstract
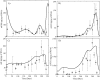
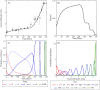
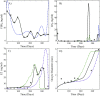
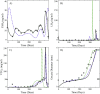
Reproduction in fishes and other vertebrates represents the timely coordination of many endocrine factors that culminate in the production of mature, viable gametes. In recent years there has been rapid growth in understanding fish reproductive biology, which has been motivated in part by recognition of the potential effects that climate change, habitat destruction and contaminant exposure can have on natural and cultured fish populations. New approaches to understanding the impacts of these stressors are being developed that require a systems biology approach with more biologically accurate and detailed mathematical models. We have developed a multi-scale mathematical model of the female rainbow trout hypothalamus-pituitary-ovary-liver axis to use as a tool to help understand the functioning of the system and for extrapolation of laboratory findings of stressor impacts on specific components of the axis. The model describes the essential endocrine components of the female rainbow trout reproductive axis. The model also describes the stage specific growth of maturing oocytes within the ovary and permits the presence of sub-populations of oocytes at different stages of development. Model formulation and parametrization was largely based on previously published in vivo and in vitro data in rainbow trout and new data on the synthesis of gonadotropins in the pituitary. Model predictions were validated against several previously published data sets for annual changes in gonadotropins and estradiol in rainbow trout. Estimates of select model parameters can be obtained from in vitro assays using either quantitative (direct estimation of rate constants) or qualitative (relative change from control values) approaches. This is an important aspect of mathematical models as in vitro, cell-based assays are expected to provide the bulk of experimental data for future risk assessments and will require quantitative physiological models to extrapolate across biological scales.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
-
- Nagahama Y, Yoshikuni M, Yamashita M, Tanaka M. Regulation of Oocyte Maturation in Fish. Fish Physiology. 1994;13:393–439.
-
- Yaron Z, Gur G, Melamed P, Rosenfeld H, Elizur A, Levavi-Sivan B. Regulation of Rish Gonadotropins. International Review of Cytology. 2003;225:131–185. - PubMed
-
- Swanson P. Salmon gonadotropins: Reconciling old and new ideas. In: Proceedings of the fourth international symposium on the reproductive physiology of fish; 1991. p. 2–7.
-
- Tyler C, Sumpter J. Oocyte growth and development in teleosts. Reviews in Fish Biology and Fisheries. 1996;6:287–318.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

