Increased microvascular permeability in mice lacking Epac1 (Rapgef3)
- PMID: 27096875
- PMCID: PMC5073050
- DOI: 10.1111/apha.12697
Increased microvascular permeability in mice lacking Epac1 (Rapgef3)
Abstract
Aim: Maintenance of the blood and extracellular volume requires tight control of endothelial macromolecule permeability, which is regulated by cAMP signalling. This study probes the role of the cAMP mediators rap guanine nucleotide exchange factor 3 and 4 (Epac1 and Epac2) for in vivo control of microvascular macromolecule permeability under basal conditions.
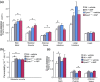
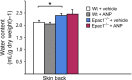
Methods: Epac1-/- and Epac2-/- C57BL/6J mice were produced and compared with wild-type mice for transvascular flux of radio-labelled albumin in skin, adipose tissue, intestine, heart and skeletal muscle. The transvascular leakage was also studied by dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI) using the MRI contrast agent Gadomer-17 as probe.
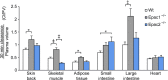
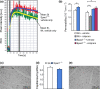
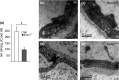
Results: Epac1-/- mice had constitutively increased transvascular macromolecule transport, indicating Epac1-dependent restriction of baseline permeability. In addition, Epac1-/- mice showed little or no enhancement of vascular permeability in response to atrial natriuretic peptide (ANP), whether probed with labelled albumin or Gadomer-17. Epac2-/- and wild-type mice had similar basal and ANP-stimulated clearances. Ultrastructure analysis revealed that Epac1-/- microvascular interendothelial junctions had constitutively less junctional complex.
Conclusion: Epac1 exerts a tonic inhibition of in vivo basal microvascular permeability. The loss of this tonic action increases baseline permeability, presumably by reducing the interendothelial permeability resistance. Part of the action of ANP to increase permeability in wild-type microvessels may involve inhibition of the basal Epac1-dependent activity.
Keywords: Epac deletion (mouse); Rapgef; atrial natriuretic peptide; cAMP; endothelial junction; microvascular permeability (in vivo).
© 2016 The Authors. Acta Physiologica published by John Wiley & Sons Ltd on behalf of Scandinavian Physiological Society.
Figures

Comment in
-
Epac1 - a tonic stabilizer of the endothelial barrier.Acta Physiol (Oxf). 2017 Feb;219(2):339-340. doi: 10.1111/apha.12738. Epub 2016 Jul 15. Acta Physiol (Oxf). 2017. PMID: 27332548 No abstract available.
References
-
- Christensen, A.E. , Selheim, F. , de Rooij, J. , Dremier, S. , Schwede, F. , Dao, K.K. , Martinez, A. , Maenhaut, C. , Bos, J.L. , Genieser, H.G. & Doskeland, S.O. 2003. cAMP analog mapping of Epac1 and cAMP kinase. Discriminating analogs demonstrate that Epac and cAMP kinase act synergistically to promote PC‐12 cell neurite extension. J Biol Chem 278, 35394–35402. - PubMed
-
- Cullere, X. , Shaw, S.K. , Andersson, L. , Hirahashi, J. , Luscinskas, F.W. & Mayadas, T.N. 2005. Regulation of vascular endothelial barrier function by Epac, a cAMP‐activated exchange factor for Rap GTPase. Blood 105, 1950–1955. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

