Beta-Adrenoceptor Stimulation Reveals Ca2+ Waves and Sarcoplasmic Reticulum Ca2+ Depletion in Left Ventricular Cardiomyocytes from Post-Infarction Rats with and without Heart Failure
- PMID: 27096943
- PMCID: PMC4838269
- DOI: 10.1371/journal.pone.0153887
Beta-Adrenoceptor Stimulation Reveals Ca2+ Waves and Sarcoplasmic Reticulum Ca2+ Depletion in Left Ventricular Cardiomyocytes from Post-Infarction Rats with and without Heart Failure
Abstract
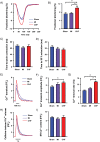
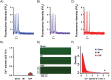
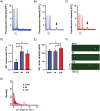
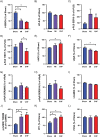
Abnormal cellular Ca2+ handling contributes to both contractile dysfunction and arrhythmias in heart failure. Reduced Ca2+ transient amplitude due to decreased sarcoplasmic reticulum Ca2+ content is a common finding in heart failure models. However, heart failure models also show increased propensity for diastolic Ca2+ release events which occur when sarcoplasmic reticulum Ca2+ content exceeds a certain threshold level. Such Ca2+ release events can initiate arrhythmias. In this study we aimed to investigate if both of these aspects of altered Ca2+ homeostasis could be found in left ventricular cardiomyocytes from rats with different states of cardiac function six weeks after myocardial infarction when compared to sham-operated controls. Video edge-detection, whole-cell Ca2+ imaging and confocal line-scan imaging were used to investigate cardiomyocyte contractile properties, Ca2+ transients and Ca2+ waves. In baseline conditions, i.e. without beta-adrenoceptor stimulation, cardiomyocytes from rats with large myocardial infarction, but without heart failure, did not differ from sham-operated animals in any of these aspects of cellular function. However, when exposed to beta-adrenoceptor stimulation, cardiomyocytes from both non-failing and failing rat hearts showed decreased sarcoplasmic reticulum Ca2+ content, decreased Ca2+ transient amplitude, and increased frequency of Ca2+ waves. These results are in line with a decreased threshold for diastolic Ca2+ release established by other studies. In the present study, factors that might contribute to a lower threshold for diastolic Ca2+ release were increased THR286 phosphorylation of Ca2+/calmodulin-dependent protein kinase II and increased protein phosphatase 1 abundance. In conclusion, this study demonstrates both decreased sarcoplasmic reticulum Ca2+ content and increased propensity for diastolic Ca2+ release events in ventricular cardiomyocytes from rats with heart failure after myocardial infarction, and that these phenomena are also found in rats with large myocardial infarctions without heart failure development. Importantly, beta-adrenoceptor stimulation is necessary to reveal these perturbations in Ca2+ handling after a myocardial infarction.
Conflict of interest statement
Figures


References
-
- Mukharji J, Rude RE, Poole WK, Gustafson N, Thomas LJ Jr., Strauss HW, et al. Risk factors for sudden death after acute myocardial infarction: two-year follow-up. The American journal of cardiology. 1984;54(1):31–6. . - PubMed
-
- Gomez AM, Valdivia HH, Cheng H, Lederer MR, Santana LF, Cannell MB, et al. Defective excitation-contraction coupling in experimental cardiac hypertrophy and heart failure. Science. 1997;276(5313):800–6. . - PubMed
-
- Kubalova Z, Terentyev D, Viatchenko-Karpinski S, Nishijima Y, Gyorke I, Terentyeva R, et al. Abnormal intrastore calcium signaling in chronic heart failure. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(39):14104–9. 10.1073/pnas.0504298102 - DOI - PMC - PubMed
-
- Ahmmed GU, Dong PH, Song G, Ball NA, Xu Y, Walsh RA, et al. Changes in Ca(2+) cycling proteins underlie cardiac action potential prolongation in a pressure-overloaded guinea pig model with cardiac hypertrophy and failure. Circulation research. 2000;86(5):558–70. . - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

