Targeting the MIR34C-5p-ATG4B-autophagy axis enhances the sensitivity of cervical cancer cells to pirarubicin
- PMID: 27097054
- PMCID: PMC4990997
- DOI: 10.1080/15548627.2016.1173798
Targeting the MIR34C-5p-ATG4B-autophagy axis enhances the sensitivity of cervical cancer cells to pirarubicin
Abstract
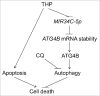
Pirarubicin (THP) is a newer generation anthracycline anticancer drug. In the clinic, THP and THP-based combination therapies have been demonstrated to be effective against various tumors without severe side effects. However, previous clinical studies have shown that most patients with cervical cancer are not sensitive to THP treatment, and the associated mechanisms are not clear. Consistent with the clinical study, we confirmed that cervical cancer cells were resistant to THP in vitro and in vivo. Our data demonstrated that THP induced a protective macroautophagy/autophagy response in cervical cancer cells, and suppression of this autophagy dramatically enhanced the cytotoxicity of THP. By scanning the mRNA level change of autophagy-related genes, we found that the upregulation of ATG4B (autophagy-related 4B cysteine peptidase) plays an important role in THP-induced autophagy. Moreover, THP increased the mRNA level of ATG4B in cervical cancer cells by promoting mRNA stability without influencing its transcription. Furthermore, THP triggered a downregulation of MIR34C-5p, which was associated with the upregulation of ATG4B and autophagy induction. Overexpression of MIR34C-5p significantly decreased the level of ATG4B and attenuated autophagy, accompanied by enhanced cell death and apoptosis in THP-treated cervical cancer cells. These results for the first time reveal the presence of a MIR34C-5p-ATG4B-autophagy signaling axis in THP-treated cervical cancer cells in vitro and in vivo, and the axis, at least partially, accounts for the THP nonsensitivity in cervical cancer patients. This study may provide a new insight for improving the chemotherapeutic effect of THP, which may be beneficial to the further clinical application of THP in cervical cancer treatment.
Keywords: ATG4B; MIR34C-5p; autophagy; cervical cancer; pirarubicin.
Figures




Similar articles
-
Berberine increases the killing effect of pirarubicin on HCC cells by inhibiting ATG4B-autophagy pathway.Exp Cell Res. 2024 Jun 1;439(1):114094. doi: 10.1016/j.yexcr.2024.114094. Epub 2024 May 13. Exp Cell Res. 2024. PMID: 38750718
-
SLC27A4 regulate ATG4B activity and control reactions to chemotherapeutics-induced autophagy in human lung cancer cells.Tumour Biol. 2016 May;37(5):6943-52. doi: 10.1007/s13277-015-4587-4. Epub 2015 Dec 11. Tumour Biol. 2016. PMID: 26662804
-
[Sodium valprovate suppresses autophagy in SH-SY5Y cells via activating miR-34c-5p/ATG4B signaling pathway].Nan Fang Yi Ke Da Xue Xue Bao. 2018 Dec 30;38(12):1415-1420. doi: 10.12122/j.issn.1673-4254.2018.12.03. Nan Fang Yi Ke Da Xue Xue Bao. 2018. PMID: 30613007 Free PMC article. Chinese.
-
Targeting Atg4B for cancer therapy: Chemical mediators.Eur J Med Chem. 2021 Jan 1;209:112917. doi: 10.1016/j.ejmech.2020.112917. Epub 2020 Oct 11. Eur J Med Chem. 2021. PMID: 33077263 Review.
-
On ATG4B as Drug Target for Treatment of Solid Tumours-The Knowns and the Unknowns.Cells. 2019 Dec 24;9(1):53. doi: 10.3390/cells9010053. Cells. 2019. PMID: 31878323 Free PMC article. Review.
Cited by
-
Deacetylation of ATG4B promotes autophagy initiation under starvation.Sci Adv. 2022 Aug 5;8(31):eabo0412. doi: 10.1126/sciadv.abo0412. Epub 2022 Aug 3. Sci Adv. 2022. PMID: 35921421 Free PMC article.
-
Knockdown ATG4C inhibits gliomas progression and promotes temozolomide chemosensitivity by suppressing autophagic flux.J Exp Clin Cancer Res. 2019 Jul 10;38(1):298. doi: 10.1186/s13046-019-1287-8. J Exp Clin Cancer Res. 2019. PMID: 31291988 Free PMC article.
-
Autophagy mediated immune response regulation and drug resistance in cancer.Mol Biol Rep. 2025 May 22;52(1):492. doi: 10.1007/s11033-025-10573-5. Mol Biol Rep. 2025. PMID: 40402380 Review.
-
To be or not to be: navigating the influence of MicroRNAs on cervical cancer cell death.Cancer Cell Int. 2025 Apr 18;25(1):153. doi: 10.1186/s12935-025-03786-y. Cancer Cell Int. 2025. PMID: 40251577 Free PMC article. Review.
-
Targeting the autophagy promoted antitumor effect of T-DM1 on HER2-positive gastric cancer.Cell Death Dis. 2021 Mar 17;12(4):288. doi: 10.1038/s41419-020-03349-1. Cell Death Dis. 2021. PMID: 33731670 Free PMC article.
References
-
- Nowak R, Tarasiuk J. Anthraquinone antitumour agents, doxorubicin, pirarubicin and benzoperimidine BP1, trigger caspase-3/caspase-8-dependent apoptosis of leukaemia sensitive HL60 and resistant HL60/VINC and HL60/DOX cells. Anticancer Drugs 2012; 23:380-92; PMID:22198116; http://dx.doi.org/10.1097/CAD.0b013e32834f8ab4 - DOI - PubMed
-
- Liu S, Hou J, Zhang H, Wu Y, Hu M, Zhang L, Xu J, Na R, Jiang H, Ding Q. The evaluation of the risk factors for non-muscle invasive bladder cancer (NMIBC) recurrence after transurethral resection (TURBt) in Chinese population. PLoS One 2015; 10:e0123617; PMID:25849552; http://dx.doi.org/10.1371/journal.pone.0123617 - DOI - PMC - PubMed
-
- Kong D, Ma S, Liang B, Yi H, Zhao Y, Xin R, Cui L, Jia L, Liu X, Liu X. The different regulatory effects of p53 status on multidrug resistance are determined by autophagy in ovarian cancer cells. Biomed Pharmacother 2012; 66:271-8; PMID:22564245; http://dx.doi.org/10.1016/j.biopha.2011.12.002 - DOI - PubMed
-
- Gu X, Jia S, Wei W, Zhang W-H. Neoadjuvant chemotherapy of breast cancer with pirarubicin versus epirubicin in combination with cyclophosphamide and docetaxel. Tumour Biol 2015; 36:5529-35; PMID:25682286; http://dx.doi.org/10.1007/s13277-015-3221-9 - DOI - PubMed
-
- Kato T, Nishimura H, Umezu J, Takeuchi S, Kanazawa K, Inoue H, Suzuki M, Hirono M, Suzuki T, Okajima H, et al.. [Phase II study of 4′-O-tetrahydropyranyl-adriamycin (THP-ADM) in patients with gynecological cancer]. Gan To Kagaku Ryoho 1985; 12:1962-7; PMID:4051511 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
