XAF1 promotes neuroblastoma tumor suppression and is required for KIF1Bβ-mediated apoptosis
- PMID: 27097110
- PMCID: PMC5085151
- DOI: 10.18632/oncotarget.8748
XAF1 promotes neuroblastoma tumor suppression and is required for KIF1Bβ-mediated apoptosis
Abstract
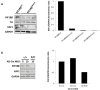
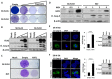
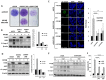
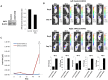
Neuroblastoma is an aggressive, relapse-prone childhood tumor of the sympathetic nervous system. Current treatment modalities do not fully exploit the genetic basis between the different molecular subtypes and little is known about the targets discovered in recent mutational and genetic studies. Neuroblastomas with poor prognosis are often characterized by 1p36 deletion, containing the kinesin gene KIF1B. Its beta isoform, KIF1Bβ, is required for NGF withdrawal-dependent apoptosis, mediated by the induction of XIAP-associated Factor 1 (XAF1). Here, we showed that XAF1 low expression correlates with poor survival and disease status. KIF1Bβ deletion results in loss of XAF1 expression, suggesting that XAF1 is indeed a downstream target of KIF1Bβ. XAF1 silencing protects from NGF withdrawal and from KIF1Bβ-mediated apoptosis. Overexpression of XAF1 impairs tumor progression whereas knockdown of XAF1 promotes tumor growth, suggesting that XAF1 may be a candidate tumor suppressor in neuroblastoma and its associated pathway may be important for developing future interventions.
Keywords: KIF1Bβ; XAF1; apoptosis; neuroblastoma.
Conflict of interest statement
FINANCIAL SUPPORT AND CONFLICTS OF INTEREST Zhang'e Choo and the project is supported by the National Medical Research Council, Singapore New Investigator Grant awarded to Zhi Xiong Chen. Susanne Schlisio is supported by grants from the Swedish Children Cancer Foundation and the Swedish Research Council. In addition, all authors declare that there is no conflict of interest.
Figures

References
-
- Lee S, Nakamura E, Yang H, Wei W, Linggi MS, Sajan MP, et al. Neuronal apoptosis linked to EglN3 prolyl hydroxylase and familial pheochromocytoma genes: developmental culling and cancer. Cancer Cell. 2005;8:155–67. doi: 10.1016/j.ccr.2005.06.015. Epub 2005/08/16. [pii] S1535-6108(05)00224-2. - DOI - PubMed
-
- Sommer L, Rao M. Neural stem cells and regulation of cell number. Progress in neurobiology. 2002;66:1–18. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

