Adverse Events in Robotic Surgery: A Retrospective Study of 14 Years of FDA Data
- PMID: 27097160
- PMCID: PMC4838256
- DOI: 10.1371/journal.pone.0151470
Adverse Events in Robotic Surgery: A Retrospective Study of 14 Years of FDA Data
Abstract
Background: Use of robotic systems for minimally invasive surgery has rapidly increased during the last decade. Understanding the causes of adverse events and their impact on patients in robot-assisted surgery will help improve systems and operational practices to avoid incidents in the future.
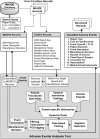
Methods: By developing an automated natural language processing tool, we performed a comprehensive analysis of the adverse events reported to the publicly available MAUDE database (maintained by the U.S. Food and Drug Administration) from 2000 to 2013. We determined the number of events reported per procedure and per surgical specialty, the most common types of device malfunctions and their impact on patients, and the potential causes for catastrophic events such as patient injuries and deaths.
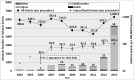
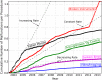
Results: During the study period, 144 deaths (1.4% of the 10,624 reports), 1,391 patient injuries (13.1%), and 8,061 device malfunctions (75.9%) were reported. The numbers of injury and death events per procedure have stayed relatively constant (mean = 83.4, 95% confidence interval (CI), 74.2-92.7 per 100,000 procedures) over the years. Surgical specialties for which robots are extensively used, such as gynecology and urology, had lower numbers of injuries, deaths, and conversions per procedure than more complex surgeries, such as cardiothoracic and head and neck (106.3 vs. 232.9 per 100,000 procedures, Risk Ratio = 2.2, 95% CI, 1.9-2.6). Device and instrument malfunctions, such as falling of burnt/broken pieces of instruments into the patient (14.7%), electrical arcing of instruments (10.5%), unintended operation of instruments (8.6%), system errors (5%), and video/imaging problems (2.6%), constituted a major part of the reports. Device malfunctions impacted patients in terms of injuries or procedure interruptions. In 1,104 (10.4%) of all the events, the procedure was interrupted to restart the system (3.1%), to convert the procedure to non-robotic techniques (7.3%), or to reschedule it (2.5%).
Conclusions: Despite widespread adoption of robotic systems for minimally invasive surgery in the U.S., a non-negligible number of technical difficulties and complications are still being experienced during procedures. Adoption of advanced techniques in design and operation of robotic surgical systems and enhanced mechanisms for adverse event reporting may reduce these preventable incidents in the future.
Conflict of interest statement
Figures

References
-
- "Annual Report 2013," Intuitive Surgical; http://phx.corporate-ir.net/External.File?item=UGFyZW50SUQ9MjIzOTk3fENoa....
-
- "The da Vinci Surgical System," Intuitive Surgical Inc.; http://www.intuitivesurgical.com/products/davinci_surgical_system/.
-
- "RIO® Robotic Arm Interactive System," MAKO Surgical Corp., http://www.makosurgical.com/physicians/products/rio.
-
- Kazanzidesf, Peter, et al. "An open-source research kit for the da Vinci® Surgical System." Robotics and Automation (ICRA), 2014 IEEE International Conference on. IEEE, 2014.
-
- Lum Mitchell JH, et al. "The RAVEN: Design and validation of a telesurgery system." The International Journal of Robotics Research 289 (2009): 1183–1197.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

