Spaceflight Activates Lipotoxic Pathways in Mouse Liver
- PMID: 27097220
- PMCID: PMC4838331
- DOI: 10.1371/journal.pone.0152877
Spaceflight Activates Lipotoxic Pathways in Mouse Liver
Erratum in
-
Correction: Spaceflight Activates Lipotoxic Pathways in Mouse Liver.PLoS One. 2016 May 4;11(5):e0155282. doi: 10.1371/journal.pone.0155282. eCollection 2016. PLoS One. 2016. PMID: 27145222 Free PMC article.
Abstract
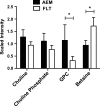
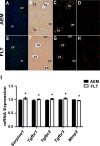
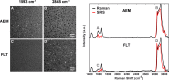
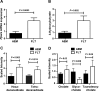
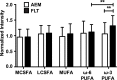
Spaceflight affects numerous organ systems in the body, leading to metabolic dysfunction that may have long-term consequences. Microgravity-induced alterations in liver metabolism, particularly with respect to lipids, remain largely unexplored. Here we utilize a novel systems biology approach, combining metabolomics and transcriptomics with advanced Raman microscopy, to investigate altered hepatic lipid metabolism in mice following short duration spaceflight. Mice flown aboard Space Transportation System -135, the last Shuttle mission, lose weight but redistribute lipids, particularly to the liver. Intriguingly, spaceflight mice lose retinol from lipid droplets. Both mRNA and metabolite changes suggest the retinol loss is linked to activation of PPARα-mediated pathways and potentially to hepatic stellate cell activation, both of which may be coincident with increased bile acids and early signs of liver injury. Although the 13-day flight duration is too short for frank fibrosis to develop, the retinol loss plus changes in markers of extracellular matrix remodeling raise the concern that longer duration exposure to the space environment may result in progressive liver damage, increasing the risk for nonalcoholic fatty liver disease.
Conflict of interest statement
Figures


References
-
- Da Silva MS, Zimmerman PM, Meguid MM, Nandi J, Ohinata K, Xu Y, et al. Anorexia in space and possible etiologies: an overview. Nutrition. 2002;18(10):805–13. Epub 2002/10/04. . - PubMed
-
- Lane HW, Gretebeck RJ, Smith SM. Nutrition, endocrinology, and body composition during space flight. Nutr Res. 1998;18(11):1923–34. Epub 2001/09/07. . - PubMed
-
- Tobin BW, Uchakin PN, Leeper-Woodford SK . Insulin secretion and sensitivity in space flight: diabetogenic effects. Nutrition. 2002;18(10):842–8. Epub 2002/10/04. . - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

