Assessment of the expression and role of the α1-nAChR subunit in efferent cholinergic function during the development of the mammalian cochlea
- PMID: 27098031
- PMCID: PMC4978794
- DOI: 10.1152/jn.01038.2015
Assessment of the expression and role of the α1-nAChR subunit in efferent cholinergic function during the development of the mammalian cochlea
Abstract
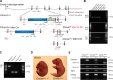
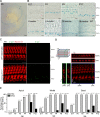
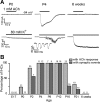
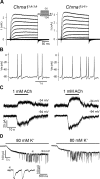
Hair cell (HC) activity in the mammalian cochlea is modulated by cholinergic efferent inputs from the brainstem. These inhibitory inputs are mediated by calcium-permeable nicotinic acetylcholine receptors (nAChRs) containing α9- and α10-subunits and by subsequent activation of calcium-dependent potassium channels. Intriguingly, mRNAs of α1- and γ-nAChRs, subunits of the "muscle-type" nAChR have also been found in developing HCs (Cai T, Jen HI, Kang H, Klisch TJ, Zoghbi HY, Groves AK. J Neurosci 35: 5870-5883, 2015; Scheffer D, Sage C, Plazas PV, Huang M, Wedemeyer C, Zhang DS, Chen ZY, Elgoyhen AB, Corey DP, Pingault V. J Neurochem 103: 2651-2664, 2007; Sinkkonen ST, Chai R, Jan TA, Hartman BH, Laske RD, Gahlen F, Sinkkonen W, Cheng AG, Oshima K, Heller S. Sci Rep 1: 26, 2011) prompting proposals that another type of nAChR is present and may be critical during early synaptic development. Mouse genetics, histochemistry, pharmacology, and whole cell recording approaches were combined to test the role of α1-nAChR subunit in HC efferent synapse formation and cholinergic function. The onset of α1-mRNA expression in mouse HCs was found to coincide with the onset of the ACh response and efferent synaptic function. However, in mouse inner hair cells (IHCs) no response to the muscle-type nAChR agonists (±)-anatoxin A, (±)-epibatidine, (-)-nicotine, or 1,1-dimethyl-4-phenylpiperazinium iodide (DMPP) was detected, arguing against the presence of an independent functional α1-containing muscle-type nAChR in IHCs. In α1-deficient mice, no obvious change of IHC efferent innervation was detected at embryonic day 18, contrary to the hyperinnervation observed at the neuromuscular junction. Additionally, ACh response and efferent synaptic activity were detectable in α1-deficient IHCs, suggesting that α1 is not necessary for assembly and membrane targeting of nAChRs or for efferent synapse formation in IHCs.
Keywords: cochlea; efferent cholinergic synapse; hair cell; synapse formation; α1-nicotinic acetylcholine receptor; α9α10-nicotinic acetylcholine receptor.
Figures


References
-
- Albuquerque EX, McIsaac RJ. Early development of acetylcholine receptors on fast and slow mammalian skeletal muscle. Life Sci 8: 409–416, 1969. - PubMed
-
- An MC, Lin W, Yang J, Dominguez B, Padgett D, Sugiura Y, Aryal P, Gould TW, Oppenheim RW, Hester ME, Kaspar BK, Ko CP, Lee KF. Acetylcholine negatively regulates development of the neuromuscular junction through distinct cellular mechanisms. Proc Natl Acad Sci USA 107: 10702–10707, 2010. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

