From molecular interaction to acute promyelocytic leukemia: Calculating leukemogenesis and remission from endogenous molecular-cellular network
- PMID: 27098097
- PMCID: PMC4838884
- DOI: 10.1038/srep24307
From molecular interaction to acute promyelocytic leukemia: Calculating leukemogenesis and remission from endogenous molecular-cellular network
Abstract
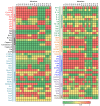
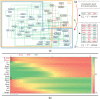
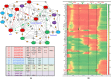
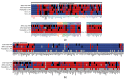
Acute promyelocytic leukemia (APL) remains the best example of a malignancy that can be cured clinically by differentiation therapy. We demonstrate that APL may emerge from a dynamical endogenous molecular-cellular network obtained from normal, non-cancerous molecular interactions such as signal transduction and translational regulation under physiological conditions. This unifying framework, which reproduces APL, normal progenitor, and differentiated granulocytic phenotypes as different robust states from the network dynamics, has the advantage to study transition between these states, i.e. critical drivers for leukemogenesis and targets for differentiation. The simulation results quantitatively reproduce microarray profiles of NB4 and HL60 cell lines in response to treatment and normal neutrophil differentiation, and lead to new findings such as biomarkers for APL and additional molecular targets for arsenic trioxide therapy. The modeling shows APL and normal states mutually suppress each other, both in "wiring" and in dynamical cooperation. Leukemogenesis and recovery under treatment may be a consequence of spontaneous or induced transitions between robust states, through "passes" or "dragging" by drug effects. Our approach rationalizes leukemic complexity and constructs a platform towards extending differentiation therapy by performing "dry" molecular biology experiments.
Figures


References
-
- National Cancer Institute, Bethesda, MD. SEER Cancer Statistics Factsheets: Leukemia. URL http://seer.cancer.gov/statfacts/html/leuks.html. (2012) (Date of access:10/02/2016).
-
- Wang Z.-Y. & Chen Z. Acute promyelocytic leukemia: from highly fatal to highly curable. Blood 111, 2505–2515 (2008). - PubMed
-
- Lo-Coco F. & Hasan S. K. Understanding the molecular pathogenesis of acute promyelocytic leukemia. Best Pract. Res. Clin. Hematol. 27, 3–9 (2014). - PubMed
-
- Kogan S. C. Mouse models of acute promyelocytic leukemia. In Pandolfi P. & Vogt P. (eds.) Acute Promyelocytic Leukemia vol. 313 of Current Topics in Microbiology and Immunology 3–29 (Springer, Berlin, 2007). - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

