Effect of age and sex on efficacy and tolerability of β blockers in patients with heart failure with reduced ejection fraction: individual patient data meta-analysis
- PMID: 27098105
- PMCID: PMC4849174
- DOI: 10.1136/bmj.i1855
Effect of age and sex on efficacy and tolerability of β blockers in patients with heart failure with reduced ejection fraction: individual patient data meta-analysis
Abstract
Objectives: To determine the efficacy and tolerability of β blockers in a broad age range of women and men with heart failure with reduced ejection fraction (HFrEF) by pooling individual patient data from placebo controlled randomised trials.
Design: Prospectively designed meta-analysis of individual patient data from patients aged 40-85 in sinus rhythm at baseline, with left ventricular ejection fraction <0.45.
Participants: 13,833 patients from 11 trials; median age 64; 24% women.
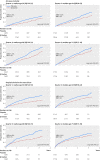
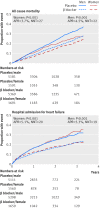
Main outcome measures: The primary outcome was all cause mortality; the major secondary outcome was admission to hospital for heart failure. Analysis was by intention to treat with an adjusted one stage Cox proportional hazards model.
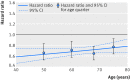
Results: Compared with placebo, β blockers were effective in reducing mortality across all ages: hazard ratios were 0.66 (95% confidence interval 0.53 to 0.83) for the first quarter of age distribution (median age 50); 0.71 (0.58 to 0.87) for the second quarter (median age 60); 0.65 (0.53 to 0.78) for the third quarter (median age 68); and 0.77 (0.64 to 0.92) for the fourth quarter (median age 75). There was no significant interaction when age was modelled continuously (P=0.1), and the absolute reduction in mortality was 4.3% over a median follow-up of 1.3 years (number needed to treat 23). Admission to hospital for heart failure was significantly reduced by β blockers, although this effect was attenuated at older ages (interaction P=0.05). There was no evidence of an interaction between treatment effect and sex in any age group. Drug discontinuation was similar regardless of treatment allocation, age, or sex (14.4% in those give β blockers, 15.6% in those receiving placebo).
Conclusion: Irrespective of age or sex, patients with HFrEF in sinus rhythm should receive β blockers to reduce the risk of death and admission to hospital.Registration PROSPERO CRD42014010012; Clinicaltrials.gov NCT00832442.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://group.bmj.com/group/rights-licensing/permissions.
Conflict of interest statement
Competing interests: All authors have completed the ICMJE uniform disclosure form (
Figures
Comment in
-
β blockers for heart failure.BMJ. 2016 Apr 20;353:i2074. doi: 10.1136/bmj.i2074. BMJ. 2016. PMID: 27099266 Free PMC article.
References
-
- Komajda M, Hanon O, Hochadel M, et al. Contemporary management of octogenarians hospitalized for heart failure in Europe: Euro Heart Failure Survey II. Eur Heart J 2009;30:478-86. 10.1093/eurheartj/ehn539 pmid:19106198. - DOI - PubMed
-
- Baumhäkel M, Müller U, Böhm M. Influence of gender of physicians and patients on guideline-recommended treatment of chronic heart failure in a cross-sectional study. Eur J Heart Fail 2009;11:299-303. 10.1093/eurjhf/hfn041 pmid:19158153. - DOI - PMC - PubMed
-
- Mehta PA, Cowie MR. Gender and heart failure: a population perspective. Heart 2006;92(Suppl 3):iii14-8. 10.1136/hrt.2005.070342 pmid:16614262. - DOI - PMC - PubMed
-
- Ali Raza J, Movahed A. Use of cardiovascular medications in the elderly. Int J Cardiol 2002;85:203-15. 10.1016/S0167-5273(02)00193-6 pmid:12208585. - DOI - PubMed
-
- Hsich EM, Piña IL. Heart failure in women: a need for prospective data. J Am Coll Cardiol 2009;54:491-8. 10.1016/j.jacc.2009.02.066 pmid:19643307. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
