Control of adult neurogenesis by programmed cell death in the mammalian brain
- PMID: 27098178
- PMCID: PMC4839132
- DOI: 10.1186/s13041-016-0224-4
Control of adult neurogenesis by programmed cell death in the mammalian brain
Abstract
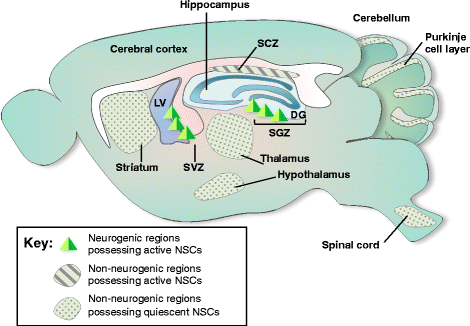
The presence of neural stem cells (NSCs) and the production of new neurons in the adult brain have received great attention from scientists and the public because of implications to brain plasticity and their potential use for treating currently incurable brain diseases. Adult neurogenesis is controlled at multiple levels, including proliferation, differentiation, migration, and programmed cell death (PCD). Among these, PCD is the last and most prominent process for regulating the final number of mature neurons integrated into neural circuits. PCD can be classified into apoptosis, necrosis, and autophagic cell death and emerging evidence suggests that all three may be important modes of cell death in neural stem/progenitor cells. However, the molecular mechanisms that regulate PCD and thereby impact the intricate balance between self-renewal, proliferation, and differentiation during adult neurogenesis are not well understood. In this comprehensive review, we focus on the extent, mechanism, and biological significance of PCD for the control of adult neurogenesis in the mammalian brain. The role of intrinsic and extrinsic factors in the regulation of PCD at the molecular and systems levels is also discussed. Adult neurogenesis is a dynamic process, and the signals for differentiation, proliferation, and death of neural progenitor/stem cells are closely interrelated. A better understanding of how adult neurogenesis is influenced by PCD will help lead to important insights relevant to brain health and diseases.
Keywords: Adult neurogenesis; Apoptosis; Autophagy; Necrosis; Neural stem cells; Neuroblasts; Programmed cell death.
Figures

References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

