Progress and potential of non-inhibitory small molecule chaperones for the treatment of Gaucher disease and its implications for Parkinson disease
- PMID: 27098312
- PMCID: PMC5381920
- DOI: 10.1080/14789450.2016.1174583
Progress and potential of non-inhibitory small molecule chaperones for the treatment of Gaucher disease and its implications for Parkinson disease
Abstract
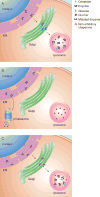
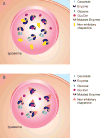
Gaucher disease, caused by pathological mutations GBA1, encodes the lysosome-resident enzyme glucocerebrosidase, which cleaves glucosylceramide into glucose and ceramide. In Gaucher disease, glucocerebrosidase deficiency leads to lysosomal accumulation of substrate, primarily in cells of the reticulo-endothelial system. Gaucher disease has broad clinical heterogeneity, and mutations in GBA1 are a risk factor for the development of different synucleinopathies. Insights into the cell biology and biochemistry of glucocerebrosidase have led to new therapeutic approaches for Gaucher disease including small chemical chaperones. Such chaperones facilitate proper enzyme folding and translocation to lysosomes, thereby preventing premature breakdown of the enzyme in the proteasome. This review discusses recent progress in developing chemical chaperones as a therapy for Gaucher disease, with implications for the treatment of synucleinopathies. It focuses on the development of non-inhibitory glucocerebrosidase chaperones and their therapeutic advantages over inhibitory chaperones, as well as the challenges involved in identifying and validating chemical chaperones.
Keywords: Gaucher disease; Lysosomal storage diseases; Parkinson disease; chemical chaperone; glucocerebrosidase; high throughput screening; synculeinopathies.
Figures
Similar articles
-
Glucocerebrosidase and its relevance to Parkinson disease.Mol Neurodegener. 2019 Aug 29;14(1):36. doi: 10.1186/s13024-019-0336-2. Mol Neurodegener. 2019. PMID: 31464647 Free PMC article. Review.
-
A new glucocerebrosidase-deficient neuronal cell model provides a tool to probe pathophysiology and therapeutics for Gaucher disease.Dis Model Mech. 2016 Jul 1;9(7):769-78. doi: 10.1242/dmm.024588. Epub 2016 May 19. Dis Model Mech. 2016. PMID: 27482815 Free PMC article.
-
High-throughput screening for small-molecule stabilizers of misfolded glucocerebrosidase in Gaucher disease and Parkinson's disease.Proc Natl Acad Sci U S A. 2024 Oct 15;121(42):e2406009121. doi: 10.1073/pnas.2406009121. Epub 2024 Oct 10. Proc Natl Acad Sci U S A. 2024. PMID: 39388267 Free PMC article.
-
The relationship between glucocerebrosidase mutations and Parkinson disease.J Neurochem. 2016 Oct;139 Suppl 1(Suppl Suppl 1):77-90. doi: 10.1111/jnc.13385. Epub 2016 Feb 10. J Neurochem. 2016. PMID: 26860875 Free PMC article. Review.
-
Glucocerebrosidase is shaking up the synucleinopathies.Brain. 2014 May;137(Pt 5):1304-22. doi: 10.1093/brain/awu002. Epub 2014 Feb 14. Brain. 2014. PMID: 24531622 Free PMC article. Review.
Cited by
-
Role of β-glucosidase 2 in aberrant glycosphingolipid metabolism: model of glucocerebrosidase deficiency in zebrafish.J Lipid Res. 2019 Nov;60(11):1851-1867. doi: 10.1194/jlr.RA119000154. Epub 2019 Sep 27. J Lipid Res. 2019. PMID: 31562193 Free PMC article.
-
Selective Targeting of the Interconversion between Glucosylceramide and Ceramide by Scaffold Tailoring of Iminosugar Inhibitors.Molecules. 2019 Jan 19;24(2):354. doi: 10.3390/molecules24020354. Molecules. 2019. PMID: 30669468 Free PMC article.
-
Protein structural features predict responsiveness to pharmacological chaperone treatment for three lysosomal storage disorders.PLoS Comput Biol. 2021 Sep 16;17(9):e1009370. doi: 10.1371/journal.pcbi.1009370. eCollection 2021 Sep. PLoS Comput Biol. 2021. PMID: 34529671 Free PMC article.
-
Induced pluripotent stem cell models of lysosomal storage disorders.Dis Model Mech. 2017 Jun 1;10(6):691-704. doi: 10.1242/dmm.029009. Dis Model Mech. 2017. PMID: 28592657 Free PMC article. Review.
-
The Emerging Role of the Lysosome in Parkinson's Disease.Cells. 2020 Nov 2;9(11):2399. doi: 10.3390/cells9112399. Cells. 2020. PMID: 33147750 Free PMC article. Review.
References
-
- de Duve C. The lysosome turns fifty. Nat Cell Biol. 2005 Sep;7(9):847–9. - PubMed
-
- Luzio JP, Parkinson MD, Gray SR, Bright NA. The delivery of endocytosed cargo to lysosomes. Biochemical Society transactions. 2009 Oct;37(Pt 5):1019–21. - PubMed
-
- Mijaljica D, Prescott M, Devenish RJ. Microautophagy in mammalian cells: revisiting a 40-year-old conundrum. Autophagy. 2011 Jul;7(7):673–82. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
