Validation of a New Three-Item Self-Report Measure for Medication Adherence
- PMID: 27098408
- PMCID: PMC5071118
- DOI: 10.1007/s10461-016-1406-x
Validation of a New Three-Item Self-Report Measure for Medication Adherence
Abstract
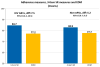
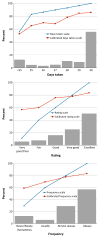
Few self-report measures of medication adherence have been rigorously developed and validated against electronic drug monitoring (EDM). Assess the validity of the 3-item self-report scale by comparing it with a contemporaneous EDM measure. We conducted an observational study in which adherence assessments were done monthly for up to 4 months for 81 patients with HIV who were taking antiretroviral medications. We report results for both HIV antiretroviral medications, and also for other, non-HIV-related medications. Raw and calibrated self-report adherence measures, electronic drug monitoring adherence measures, and sociodemographic variables. The mean age of patients was 46 years, 37 % were female, 49 % had some education beyond high school, 22 % were Black, and 22 % were Hispanic. Cronbach's alphas for the 3-item scale for HIV and non-HIV medications were 0.83 and 0.87, respectively. The mean differences (raw/uncalibrated self-report scale minus EDM) for HIV and non-HIV medications were 7.5 and 5.2 points on a 100-point scale (p < 0.05 for both). Pearson correlation coefficients between the calibrated 3-item scale and the EDM for HIV and non-HIV medications were 0.47 and 0.59, respectively. The c-statistics for the ROC curves for the calibrated scale, using cut-offs of 0.8 and 0.9 for the EDM gold standard measure to define non-adherence, were between 0.74 and 0.76 for HIV and non-HIV medications. This 3-item adherence self-report scale showed good psychometric characteristics and good construct validity when compared with an EDM standard, for both HIV and non-HIV medications. In clinical care it can be a useful first-stage screener for non-adherence. In clinical research and quality improvement settings it can be a useful tool when more complex and expensive methods such as EDM or pharmacy claims are impractical or unavailable.
Keywords: HIV; Highly active antiretroviral therapy; Medication adherence; Patient compliance; Self-report.
Figures

References
-
- Rand CS. “I took the medicine like you told me, Doctor”: self-reports of adherence with medical regimens. In: Stone AA, Turkkan JS, Bachrach CA, Jobe JB, Kurtzman HS, Cain VS, editors. The science of self-report: implications for research and practice. Vol. 1. Mahwah: Lawrence Erlbaum Associates; 2000. pp. 257–76.
-
- Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005;353(5):487–97. - PubMed
-
- Steiner JF, Prochazka AV. The assessment of refill compliance using pharmacy records: methods, validity, and applications. J Clin Epidemiol. 1997;50(1):105–16. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

