High-dose chemotherapy and autologous stem cell transplant compared with conventional chemotherapy for consolidation in newly diagnosed primary CNS lymphoma--a randomized phase III trial (MATRix)
- PMID: 27098429
- PMCID: PMC4839072
- DOI: 10.1186/s12885-016-2311-4
High-dose chemotherapy and autologous stem cell transplant compared with conventional chemotherapy for consolidation in newly diagnosed primary CNS lymphoma--a randomized phase III trial (MATRix)
Abstract
Background: Primary central nervous system lymphoma (PCNSL) is a highly aggressive Non-Hodgkin lymphoma (NHL) with rising incidence over the past 30 years in immunocompetent patients. Although outcomes have improved, PCNSL is still associated with inferior prognosis compared to systemic NHL. Many questions regarding the optimal therapeutic approach remain unanswered.
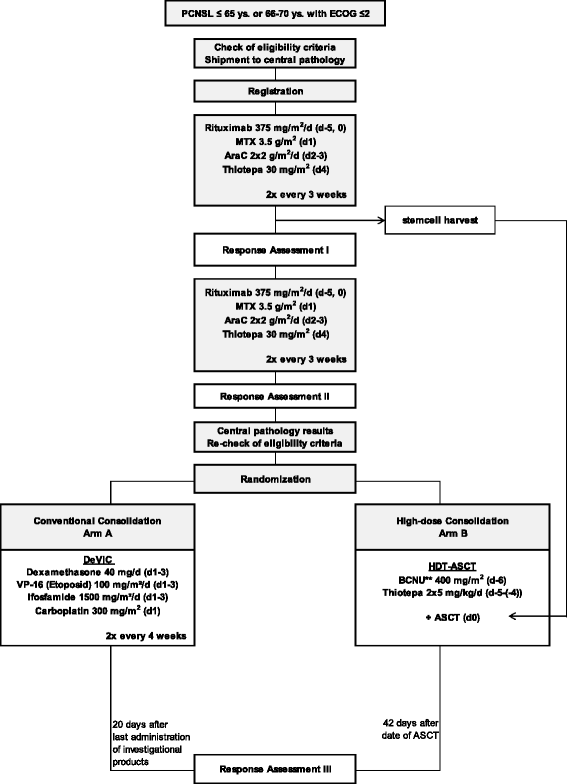
Methods/design: This is a randomized, open-label, international phase III trial with two parallel arms. We will recruit 250 patients with newly diagnosed PCNSL from approximately 35 centers within the networks of the German Cooperative PCNSL study group and the International Extranodal Lymphoma Study Group. All enrolled patients will undergo induction chemotherapy consisting of 4 cycles of rituximab 375 mg/m(2)/d (days 0 & 5), methotrexate 3.5 g/m(2) (d1), cytarabine 2 × 2 g/m(2)/d (d2-3), and thiotepa 30 mg/m(2) (d4) every 21 days. All patients will undergo stem-cell harvest after the second cycle. After 4 cycles of induction chemotherapy, patients achieving partial or complete response will be centrally randomized to 2 different consolidation treatments: (A) conventional-dose immuno chemotherapy with rituximab 375 mg/m(2) (d0), dexamethasone 40 mg/d (d1-3), etoposide 100 mg/m(2)/d (d1-3), ifosfamide 1500 mg/m(2)/d (d1-3) and carboplatin 300 mg/m(2) (d1) (R-DeVIC) or (B) high-dose chemotherapy with BCNU (or busulfan) and thiotepa followed by autologous stem cell transplantation (HCT-ASCT). The objective is to demonstrate superiority of HCT-ASCT compared to R-DeVIC with respect to progression-free survival (PFS, primary endpoint). Secondary endpoints include overall survival (OS), treatment response and treatment-related morbidities. Minimal follow-up after treatment completion is 24 months.
Discussion: The rationale for consolidation treatment in PCNSL is to eliminate residual lymphoma cells and to decrease the risk for relapse. This can be achieved by agents crossing the blood brain barrier either applied at conventional doses or at high doses requiring autologous stem cell support. HCT-ASCT has been shown to be feasible and highly effective in patients with newly-diagnosed PCNSL. However, it is unclear whether HCT-ASCT is really superior compared to conventional-dose chemotherapy after an intensified antimetabolites-based immunochemotherapy in patients with newly-diagnosed PCNSL. To answer this question, we designed this investigator initiated randomized phase III trial.
Trial registration: German clinical trials registry DRKS00005503 registered 22 April 2014 and ClinicalTrials.gov NCT02531841 registered 24 August 2015.
Keywords: Autologous stem cell transplantation (ASCT); Conventional chemotherapy; High-dose chemotherapy (HDT); Primary central nervous system lymphoma (PCNSL); Randomized controlled trial.
Figures
References
-
- Ferreri AJ, Abrey LE, Blay JY, Borisch B, Hochman J, Neuwelt EA, et al. Summary statement on primary central nervous system lymphomas from the Eighth International Conference on Malignant Lymphoma, Lugano, Switzerland, June 12 to 15, 2002. J Clin Oncol. 2003;21(12):2407–2414. doi: 10.1200/JCO.2003.01.135. - DOI - PubMed
-
- Ferreri AJ, Reni M, Foppoli M, Martelli M, Pangalis GA, Frezzato M, et al. High-dose cytarabine plus high-dose methotrexate versus high-dose methotrexate alone in patients with primary CNS lymphoma: a randomised phase 2 trial. Lancet. 2009;374(9700):1512–1520. doi: 10.1016/S0140-6736(09)61416-1. - DOI - PubMed
-
- Illerhaus G, Marks R, Ihorst G, Guttenberger R, Ostertag C, Derigs G, et al. High-dose chemotherapy with autologous stem-cell transplantation and hyperfractionated radiotherapy as first-line treatment of primary CNS lymphoma. J Clin Oncol. 2006;24(24):3865–3870. doi: 10.1200/JCO.2006.06.2117. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

