Multiwalled carbon nanotubes intratracheally instilled into the rat lung induce development of pleural malignant mesothelioma and lung tumors
- PMID: 27098557
- PMCID: PMC4946724
- DOI: 10.1111/cas.12954
Multiwalled carbon nanotubes intratracheally instilled into the rat lung induce development of pleural malignant mesothelioma and lung tumors
Abstract
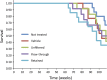
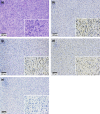
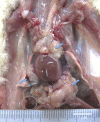
Multiwalled carbon nanotubes (MWCNT) have a fibrous structure and physical properties similar to asbestos and have been shown to induce malignant mesothelioma of the peritoneum after injection into the scrotum or peritoneal cavity in rats and mice. For human cancer risk assessment, however, data after administration of MWCNT via the airway, the exposure route that is most relevant to humans, is required. The present study was undertaken to investigate the carcinogenicity of MWCNT-N (NIKKISO) after administration to the rat lung. MWCNT-N was fractionated by passing it through a sieve with a pore size of 25 μm. The average lengths of the MWCNT were 4.2 μm before filtration and 2.6 μm in the flow-through fraction; the length of the retained MWCNT could not be determined. For the present study, 10-week-old F344/Crj male rats were divided into five groups: no treatment, vehicle control, MWCNT-N before filtration, MWCNT-N flow-through and MWCNT-N retained groups. Administration was by the trans-tracheal intrapulmonary spraying (TIPS) method. Rats were administered a total of 1 mg/rat during the initial 2 weeks of the experiment and then observed up to 109 weeks. The incidences of malignant mesothelioma and lung tumors (bronchiolo-alveolar adenomas and carcinomas) were 6/38 and 14/38, respectively, in the three groups administered MWCNT and 0/28 and 0/28, respectively, in the control groups. All malignant mesotheliomas were localized in the pericardial pleural cavity. The sieve fractions did not have a significant effect on tumor incidence. In conclusion, administration of MWCNT to the lung in the rat induces malignant mesothelioma and lung tumors.
Keywords: Intratracheal instillation; lung tumors; malignant mesothelioma; multiwalled carbon nanotubes; rat.
© 2016 The Authors. Cancer Science published by John Wiley & Sons Australia, Ltd on behalf of Japanese Cancer Association.
Figures








Similar articles
-
MWCNT-7 administered to the lung by intratracheal instillation induces development of pleural mesothelioma in F344 rats.Cancer Sci. 2019 Aug;110(8):2485-2492. doi: 10.1111/cas.14121. Epub 2019 Jul 25. Cancer Sci. 2019. PMID: 31265162 Free PMC article.
-
Comparative carcinogenicity study of a thick, straight-type and a thin, tangled-type multi-walled carbon nanotube administered by intra-tracheal instillation in the rat.Part Fibre Toxicol. 2020 Oct 15;17(1):48. doi: 10.1186/s12989-020-00382-y. Part Fibre Toxicol. 2020. PMID: 33054855 Free PMC article.
-
Carcinogenic effect of potassium octatitanate (POT) fibers in the lung and pleura of male Fischer 344 rats after intrapulmonary administration.Part Fibre Toxicol. 2019 Sep 2;16(1):34. doi: 10.1186/s12989-019-0316-2. Part Fibre Toxicol. 2019. PMID: 31477126 Free PMC article.
-
Malignant Mesothelioma and Its Non-Asbestos Causes.Arch Pathol Lab Med. 2018 Jun;142(6):753-760. doi: 10.5858/arpa.2017-0365-RA. Epub 2018 Feb 26. Arch Pathol Lab Med. 2018. PMID: 29480760 Review.
-
Iron addiction with ferroptosis-resistance in asbestos-induced mesothelial carcinogenesis: Toward the era of mesothelioma prevention.Free Radic Biol Med. 2019 Mar;133:206-215. doi: 10.1016/j.freeradbiomed.2018.10.401. Epub 2018 Oct 10. Free Radic Biol Med. 2019. PMID: 30312759 Review.
Cited by
-
Biocompatibility and Carcinogenicity of Carbon Nanotubes as Biomaterials.Nanomaterials (Basel). 2020 Feb 4;10(2):264. doi: 10.3390/nano10020264. Nanomaterials (Basel). 2020. PMID: 32033249 Free PMC article. Review.
-
Evaluating the mechanistic evidence and key data gaps in assessing the potential carcinogenicity of carbon nanotubes and nanofibers in humans.Crit Rev Toxicol. 2017 Jan;47(1):1-58. doi: 10.1080/10408444.2016.1206061. Epub 2016 Aug 18. Crit Rev Toxicol. 2017. PMID: 27537422 Free PMC article. Review.
-
Integration of inflammation, fibrosis, and cancer induced by carbon nanotubes.Nanotoxicology. 2019 Nov;13(9):1244-1274. doi: 10.1080/17435390.2019.1651920. Epub 2019 Sep 19. Nanotoxicology. 2019. PMID: 31537143 Free PMC article. Review.
-
Multiwalled carbon nanotubes activate the NLRP3 inflammasome-dependent pyroptosis in macrophages.Mol Pharmacol. 2025 May;107(5):100031. doi: 10.1016/j.molpha.2025.100031. Epub 2025 Apr 2. Mol Pharmacol. 2025. PMID: 40273527 Free PMC article.
-
Osteopontin mRNA expression by rat mesothelial cells exposed to multi-walled carbon nanotubes as a potential biomarker of chronic neoplastic transformation in vitro.Toxicol In Vitro. 2021 Jun;73:105126. doi: 10.1016/j.tiv.2021.105126. Epub 2021 Feb 27. Toxicol In Vitro. 2021. PMID: 33652123 Free PMC article.
References
-
- Donaldson K, Poland CA. Nanotoxicology: new insights into nanotubes. Nat Nanotechnol 2009; 4: 708–10. - PubMed
-
- Kohyama N, Suzuki Y. Analysis of asbestos fibers in lung parenchyma, pleural plaques, and mesothelioma tissues of North American insulation workers. Ann N Y Acad Sci 1991; 643: 27–52. - PubMed
-
- Donaldson K, Poland CA, Murphy FA, MacFarlane M, Chernova T, Schinwald A. Pulmonary toxicity of carbon nanotubes and asbestos – Similarities and differences. Adv Drug Deliv Rev 2013; 65: 2078–86. - PubMed
-
- Takagi A, Hirose A, Nishimura T et al Induction of mesothelioma in p53 + /− mouse by intraperitoneal application of multi‐wall carbon nanotube. J Toxicol Sci 2008; 33: 105–16. - PubMed
-
- Sakamoto Y, Nakae D, Fukumori N et al Induction of mesothelioma by a single intrascrotal administration of multi‐wall carbon nanotube in intact male Fischer 344 rats. J Toxicol Sci 2009; 34: 65–76. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

