Tumor necrosis factor superfamily member APRIL contributes to fibrotic scar formation after spinal cord injury
- PMID: 27098833
- PMCID: PMC4839088
- DOI: 10.1186/s12974-016-0552-4
Tumor necrosis factor superfamily member APRIL contributes to fibrotic scar formation after spinal cord injury
Abstract
Background: Fibrotic scar formation contributes to the axon growth-inhibitory environment that forms following spinal cord injury (SCI). We recently demonstrated that depletion of hematogenous macrophages led to a reduction in fibrotic scar formation and increased axon growth after SCI. These changes were associated with decreased TNFSF13 (a proliferation inducing ligand (APRIL)) expression, but the role of APRIL in fibrotic scar formation after SCI has not been directly investigated. Thus, the goal of this study was to determine the role of APRIL in fibrotic scar formation after SCI.
Methods: APRIL knockout and wild-type mice received contusive SCI and were assessed for inflammatory cytokine/chemokine expression, leukocyte infiltration, fibrotic scar formation, axon growth, and cell proliferation.
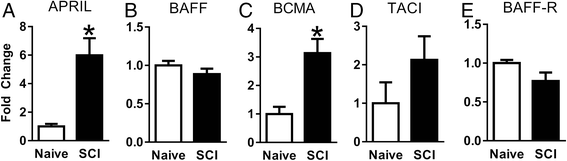
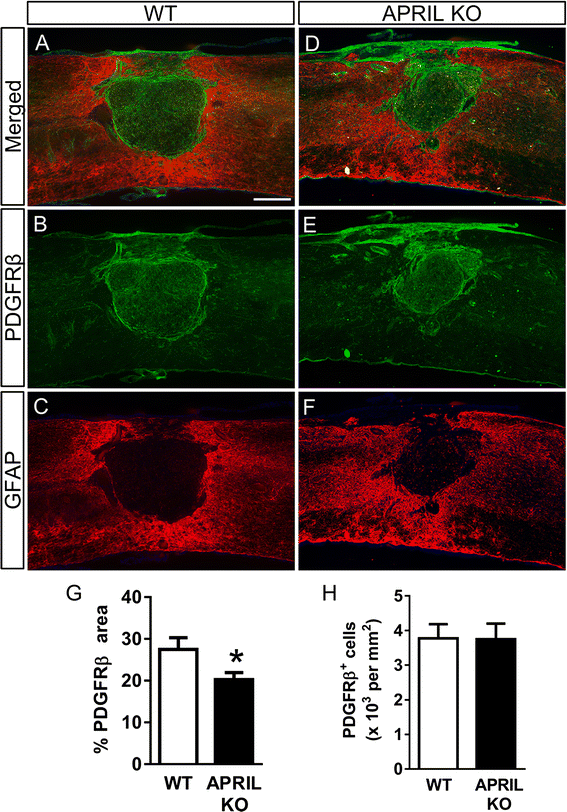
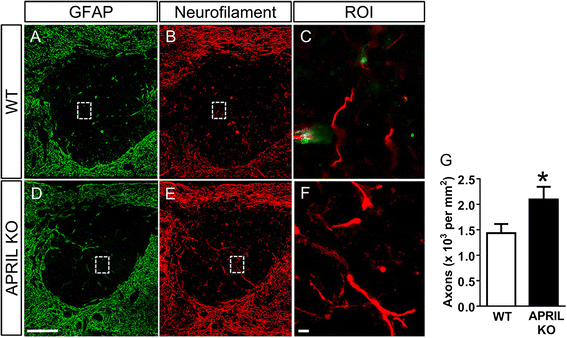
Results: Expression of APRIL and its receptor BCMA is increased following SCI, and genetic deletion of APRIL led to reduced fibrotic scar formation and increased axon growth. However, the fibrotic scar reduction in APRIL KO mice was not a result of changes in fibroblast or astrocyte proliferation. Rather, APRIL knockout mice displayed reduced TNFα and CCL2 expression and less macrophage and B cell infiltration at the injury site.
Conclusions: Our data indicate that APRIL contributes to fibrotic scar formation after SCI by mediating the inflammatory response.
Keywords: BAFF; BAFF-R; BCMA; Cell proliferation; Fibrosis; Glial scar; TACI; TNFSF13.
Figures



Similar articles
-
Hematogenous macrophage depletion reduces the fibrotic scar and increases axonal growth after spinal cord injury.Neurobiol Dis. 2015 Feb;74:114-25. doi: 10.1016/j.nbd.2014.10.024. Epub 2014 Nov 4. Neurobiol Dis. 2015. PMID: 25461258 Free PMC article.
-
SU16f inhibits fibrotic scar formation and facilitates axon regeneration and locomotor function recovery after spinal cord injury by blocking the PDGFRβ pathway.J Neuroinflammation. 2022 Apr 16;19(1):95. doi: 10.1186/s12974-022-02449-3. J Neuroinflammation. 2022. PMID: 35429978 Free PMC article.
-
Fibronectin Matrix Assembly after Spinal Cord Injury.J Neurotrauma. 2015 Aug 1;32(15):1158-67. doi: 10.1089/neu.2014.3703. Epub 2015 Mar 9. J Neurotrauma. 2015. PMID: 25492623 Free PMC article.
-
Astrocyte reactivity and astrogliosis after spinal cord injury.Neurosci Res. 2018 Jan;126:39-43. doi: 10.1016/j.neures.2017.10.004. Epub 2017 Oct 17. Neurosci Res. 2018. PMID: 29054466 Review.
-
The glial scar in spinal cord injury and repair.Neurosci Bull. 2013 Aug;29(4):421-35. doi: 10.1007/s12264-013-1358-3. Epub 2013 Jul 16. Neurosci Bull. 2013. PMID: 23861090 Free PMC article. Review.
Cited by
-
Characterization of the Expressions and m6A Methylation Modification Patterns of mRNAs and lncRNAs in a Spinal Cord Injury Rat Model.Mol Neurobiol. 2025 Jan;62(1):806-818. doi: 10.1007/s12035-024-04297-z. Epub 2024 Jun 22. Mol Neurobiol. 2025. PMID: 38907070 Free PMC article.
-
RNAi-mediated ephrin-B2 silencing attenuates astroglial-fibrotic scar formation and improves spinal cord axon growth.CNS Neurosci Ther. 2017 Oct;23(10):779-789. doi: 10.1111/cns.12723. Epub 2017 Aug 21. CNS Neurosci Ther. 2017. PMID: 28834283 Free PMC article.
-
Bone marrow mesenchymal stem cells and exercise restore motor function following spinal cord injury by activating PI3K/AKT/mTOR pathway.Neural Regen Res. 2023 May;18(5):1067-1075. doi: 10.4103/1673-5374.355762. Neural Regen Res. 2023. PMID: 36254995 Free PMC article.
-
Fibrotic Scar After Spinal Cord Injury: Crosstalk With Other Cells, Cellular Origin, Function, and Mechanism.Front Cell Neurosci. 2021 Aug 26;15:720938. doi: 10.3389/fncel.2021.720938. eCollection 2021. Front Cell Neurosci. 2021. PMID: 34539350 Free PMC article. Review.
-
Galectin-3 inhibition reduces fibrotic scarring and promotes functional recovery after spinal cord injury in mice.Cell Biosci. 2024 Oct 15;14(1):128. doi: 10.1186/s13578-024-01310-9. Cell Biosci. 2024. PMID: 39407295 Free PMC article.
References
-
- Weldon AJ, Moldovan I, Cabling MG, Hernandez EA, Hsu S, Gonzalez J, Parra A, Benitez A, Daoud N, Colburn K, Payne KJ. Surface APRIL is elevated on myeloid cells and is associated with disease activity in patients with rheumatoid arthritis. J Rheumatol. 2015;42:749–759. doi: 10.3899/jrheum.140630. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

