An Evolving Genetic Architecture Interacts with Hill-Robertson Interference to Determine the Benefit of Sex
- PMID: 27098911
- PMCID: PMC4896203
- DOI: 10.1534/genetics.116.186916
An Evolving Genetic Architecture Interacts with Hill-Robertson Interference to Determine the Benefit of Sex
Abstract
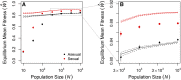
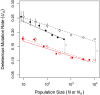
Sex is ubiquitous in the natural world, but the nature of its benefits remains controversial. Previous studies have suggested that a major advantage of sex is its ability to eliminate interference between selection on linked mutations, a phenomenon known as Hill-Robertson interference. However, those studies may have missed both important advantages and important disadvantages of sexual reproduction because they did not allow the distributions of mutational effects and interactions (i.e., the genetic architecture) to evolve. Here we investigate how Hill-Robertson interference interacts with an evolving genetic architecture to affect the evolutionary origin and maintenance of sex by simulating evolution in populations of artificial gene networks. We observed a long-term advantage of sex-equilibrium mean fitness of sexual populations exceeded that of asexual populations-that did not depend on population size. We also observed a short-term advantage of sex-sexual modifier mutations readily invaded asexual populations-that increased with population size, as was observed in previous studies. We show that the long- and short-term advantages of sex were both determined by differences between sexual and asexual populations in the evolutionary dynamics of two properties of the genetic architecture: the deleterious mutation rate ([Formula: see text]) and recombination load ([Formula: see text]). These differences resulted from a combination of selection to minimize [Formula: see text] which is experienced only by sexuals, and Hill-Robertson interference experienced primarily by asexuals. In contrast to the previous studies, in which Hill-Robertson interference had only a direct impact on the fitness advantages of sex, the impact of Hill-Robertson interference in our simulations was mediated additionally by an indirect impact on the efficiency with which selection acted to reduce [Formula: see text].
Keywords: deleterious mutation rate; evolution of sex; gene network; population size; recombination load.
Copyright © 2016 by the Genetics Society of America.
Figures





References
-
- Agrawal A. F., 2001. Sexual selection and the maintenance of sexual reproduction. Nature 411: 692–695. - PubMed
-
- Azevedo R. B. R., Lohaus R., Srinivasan S., Dang K. K., Burch C. L., 2006. Sexual reproduction selects for robustness and negative epistasis in artificial gene networks. Nature 440: 87–90. - PubMed
-
- Barton N. H., 1995. A general model for the evolution of recombination. Genet. Res. 65: 123–145. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

