Ion channel-transporter interactions
- PMID: 27098917
- PMCID: PMC5215868
- DOI: 10.3109/10409238.2016.1172553
Ion channel-transporter interactions
Abstract
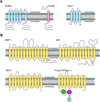
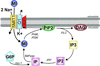
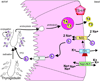
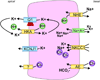
All living cells require membrane proteins that act as conduits for the regulated transport of ions, solutes and other small molecules across the cell membrane. Ion channels provide a pore that permits often rapid, highly selective and tightly regulated movement of ions down their electrochemical gradient. In contrast, active transporters can move moieties up their electrochemical gradient. The secondary active transporters (such as SLC superfamily solute transporters) achieve this by coupling uphill movement of the substrate to downhill movement of another ion, such as sodium. The primary active transporters (including H(+)/K(+)-ATPases and Na(+)/K(+)-ATPases) utilize ATP hydrolysis as an energy source to power uphill transport. It is well known that proteins in each of these classes work in concert with members of the other classes to ensure, for example, ion homeostasis, ion secretion and restoration of ion balance following action potentials. More recently, evidence is emerging of direct physical interaction between true ion channels, and some primary or secondary active transporters. Here, we review the first known members of this new class of macromolecular complexes that we term "chansporters", explore their biological roles and discuss the pathophysiological consequences of their disruption. We compare functional and/or physical interactions between the ubiquitous KCNQ1 potassium channel and various active transporters, and examine other newly discovered chansporter complexes that suggest we may be seeing the tip of the iceberg in a newly emerging signaling modality.
Keywords: ATPase; Active transport; KCNQ1; NIS; SMIT1; voltage-gated potassium channel.
Figures

References
-
- Abbott GW. Biology of the KCNQ1 Potassium Channel. [Accessed June 4, 2014];New Journal of Science. 2014 2014:1–26. Available at: http://www.hindawi.com/journals/njos/2014/237431/
-
- Abbott GW, et al. KCNQ1, KCNE2, and Na+-coupled solute transporters form reciprocally regulating complexes that affect neuronal excitability. [Accessed May 31, 2014];Science signaling. 2014 7(315):ra22. Available at: http://www.ncbi.nlm.nih.gov/pubmed/24595108. - PMC - PubMed
-
- Abbott GW, et al. MiRP2 Forms Potassium Channels in Skeletal Muscle with Kv3 . 4 and Is Associated with Periodic Paralysis. Channels. 2001;104:217–231. - PubMed
-
- Allison JH, Stewart MA. Reduced Brain Inositol in Lithium-treated Rats. Nature New Biology. 1971;233(43):267–268. Available at: http://www.nature.com/doifinder/10.1038/newbio233267a0. - DOI - PubMed
-
- Angelo K, et al. KCNE5 induces time- and voltage-dependent modulation of the KCNQ1 current. Biophysical journal. 2002;83(4):1997–2006. Available at: http://dx.doi.org/10.1016/S0006-3495(02)73961-1. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
