The minimum required level of donor chimerism in hereditary hemophagocytic lymphohistiocytosis
- PMID: 27099148
- PMCID: PMC5291300
- DOI: 10.1182/blood-2015-12-684498
The minimum required level of donor chimerism in hereditary hemophagocytic lymphohistiocytosis
Abstract
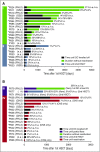
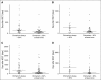
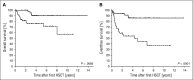
Reduced-intensity conditioning has improved survival after hematopoietic stem cell transplantation (HSCT) for hemophagocytic lymphohistiocytosis (HLH) at the cost of more frequent mixed chimerism. The minimum level of donor chimerism (DC) required to prevent HLH reactivation in humans remains to be determined. In a multicenter retrospective study, 103 patients transplanted for hereditary HLH (2000-2013) and DC permanently or transiently <75% (overall, CD3(+), CD56(+)) were analyzed regarding DC, specific immunologic function, occurrence of systemic reactivations (≥5/8 HLH criteria), partial systemic flares (<5 criteria and HLH-directed treatment), isolated central nervous system reactivations, and management. Recurrence was reported in 18 patients (systemic reactivation n = 11, partial flare n = 3, isolated central nervous system reactivation n = 4). Ten events occurred during profound immune suppression before day 180 (median DC, 10%; range, 1-100%; CD3(+) if available, otherwise overall DC), which renders a differentiation between secondary post-HSCT HLH and HLH related to the genetic defect difficult. Eight events occurred between 0.5 and 6.7 years post-HSCT (median DC, 13%; range, 0-30%). In 5 patients, overall and lineage-specific DC were ≤10% for >6 months (median, 5.1; range, 1.1-10 years) without reactivation. A second HSCT was performed in 18 patients (median, DC 4%; range, 0-19%). Death from reactivation occurred in 4 patients (22% of recurrences). Six patients died of transplant complications following a second HSCT (33% of second HSCT). We conclude that a DC >20%-30% is protective against late reactivation. Lower levels do not, however, inescapably result in recurrences. The decision for or against second HSCT must be based on a thorough risk assessment.
© 2016 by The American Society of Hematology.
Figures


References
-
- Henter JI, Horne A, Aricó M, et al. HLH-2004: diagnostic and therapeutic guidelines for hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer. 2007;48(2):124–131. - PubMed
-
- Janka GE, Lehmberg K. Hemophagocytic syndromes—an update. Blood Rev. 2014;28(4):135–142. - PubMed
-
- Baker KS, Filipovich AH, Gross TG, et al. Unrelated donor hematopoietic cell transplantation for hemophagocytic lymphohistiocytosis. Bone Marrow Transplant. 2008;42(3):175–180. - PubMed
-
- Horne A, Janka G, Maarten Egeler R, et al. Histiocyte Society. Haematopoietic stem cell transplantation in haemophagocytic lymphohistiocytosis. Br J Haematol. 2005;129(5):622–630. - PubMed
-
- Ouachée-Chardin M, Elie C, de Saint Basile G, et al. Hematopoietic stem cell transplantation in hemophagocytic lymphohistiocytosis: a single-center report of 48 patients. Pediatrics. 2006;117(4):e743–e750. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

