Lentiviral hematopoietic stem cell gene therapy for X-linked severe combined immunodeficiency
- PMID: 27099176
- PMCID: PMC5557273
- DOI: 10.1126/scitranslmed.aad8856
Lentiviral hematopoietic stem cell gene therapy for X-linked severe combined immunodeficiency
Erratum in
-
Erratum for the Research Article: "Lentiviral hematopoietic stem cell gene therapy for X-linked severe combined immunodeficiency" by S. S. De Ravin, X. Wu, S. Moir, S. Anaya-O'Brien, N. Kwatemaa, P. Littel, N. Theobald, U. Choi, L. Su, M. Marquesen, D. Hilligoss, J. Lee, C. M. Buckner, K. A. Zarember, G. O'Connor, D. McVicar, D. Kuhns, R. E. Throm, S. Zhou, L. D. Notarangelo, I. C. Hanson, M. J. Cowan, E. Kang, C. Hadigan, M. Meagher, J. T. Gray, B. P. Sorrentino, H. L. Malech.Sci Transl Med. 2016 Jun 1;8(341):341er5. doi: 10.1126/scitranslmed.aag1383. Sci Transl Med. 2016. PMID: 27252172 No abstract available.
Abstract
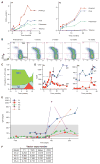
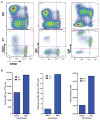
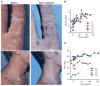
X-linked severe combined immunodeficiency (SCID-X1) is a profound deficiency of T, B, and natural killer (NK) cell immunity caused by mutations inIL2RGencoding the common chain (γc) of several interleukin receptors. Gamma-retroviral (γRV) gene therapy of SCID-X1 infants without conditioning restores T cell immunity without B or NK cell correction, but similar treatment fails in older SCID-X1 children. We used a lentiviral gene therapy approach to treat five SCID-X1 patients with persistent immune dysfunction despite haploidentical hematopoietic stem cell (HSC) transplant in infancy. Follow-up data from two older patients demonstrate that lentiviral vector γc transduced autologous HSC gene therapy after nonmyeloablative busulfan conditioning achieves selective expansion of gene-marked T, NK, and B cells, which is associated with sustained restoration of humoral responses to immunization and clinical improvement at 2 to 3 years after treatment. Similar gene marking levels have been achieved in three younger patients, albeit with only 6 to 9 months of follow-up. Lentiviral gene therapy with reduced-intensity conditioning appears safe and can restore humoral immune function to posthaploidentical transplant older patients with SCID-X1.
Copyright © 2016, American Association for the Advancement of Science.
Conflict of interest statement
Figures



References
-
- Neven B, Leroy S, Decaluwe H, Le Deist F, Picard C, Moshous D, Mahlaoui N, Debré M, Casanova J-L, Dal Cortivo L, Madec Y, Hacein-Bey-Abina S, de Saint Basile G, de Villartay J-P, Blanche S, Cavazzana-Calvo M, Fischer A. Long-term outcome after hematopoietic stem cell transplantation of a single-center cohort of 90 patients with severe combined immunodeficiency. Blood. 2009;113:4114–4124. - PubMed
-
- Pai SY, Logan BR, Griffith LM, Buckley RH, Parrott RE, Dvorak CC, Kapoor N, Hanson IC, Filipovich AH, Jyonouchi S, Sullivan KE, Small TN, Burroughs L, Skoda-Smith S, Haight AE, Grizzle A, Pulsipher MA, Chan KW, Fuleihan RL, Haddad E, Loechelt B, Aquino VM, Gillio A, Davis J, Knutsen A, Smith AR, Moore TB, Schroeder ML, Goldman FD, Connelly JA, Porteus MH, Xiang Q, Shearer WT, Fleisher TA, Kohn DB, Puck JM, Notarangelo LD, Cowan MJ, O’Reilly RJ. Transplantation outcomes for severe combined immunodeficiency, 2000–2009. N Engl J Med. 2014;371:434–446. - PMC - PubMed
-
- Hacein-Bey-Abina S, Hauer J, Lim A, Picard C, Wang GP, Berry CC, Martinache C, Rieux-Laucat F, Latour S, Belohradsky BH, Leiva L, Sorensen R, Debré M, Laurent Casanova J, Blanche S, Durandy A, Bushman FD, Fischer A, Cavazzana-Calvo M. Efficacy of gene therapy for X-linked severe combined immunodeficiency. N Engl J Med. 2010;363:355–364. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

