Xenopus: leaping forward in kidney organogenesis
- PMID: 27099217
- PMCID: PMC5074909
- DOI: 10.1007/s00467-016-3372-y
Xenopus: leaping forward in kidney organogenesis
Abstract
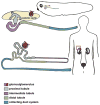
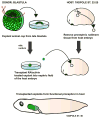
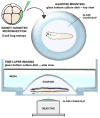
While kidney donations stagnate, the number of people in need of kidney transplants continues to grow. Although transplanting culture-grown organs is years away, pursuing the engineering of the kidney de novo is a valid means of closing the gap between the supply and demand of kidneys for transplantation. The structural organization of a mouse kidney is similar to that of humans. Therefore, mice have traditionally served as the primary model system for the study of kidney development. The mouse is an ideal model organism for understanding the complexity of the human kidney. Nonetheless, the elaborate structure of the mammalian kidney makes the discovery of new therapies based on de novo engineered kidneys more challenging. In contrast to mammals, amphibians have a kidney that is anatomically less complex and develops faster. Given that analogous genetic networks regulate the development of mammalian and amphibian nephric organs, using embryonic kidneys of Xenopus laevis (African clawed frog) to analyze inductive cell signaling events and morphogenesis has many advantages. Pioneering work that led to the ability to generate kidney organoids from embryonic cells was carried out in Xenopus. In this review, we discuss how Xenopus can be utilized to compliment the work performed in mammalian systems to understand kidney development.
Keywords: Development; Induction; Kidney; Nephron; Organoid; Pronephros; Xenopus.
Conflict of interest statement
Figures
References
-
- Moody SA. Fates of the blastomeres of the 16-cell stage Xenopus embryo. Dev Biol. 1987;119:560–578. - PubMed
-
- Moody SA. Fates of the blastomeres of the 32-cell stage Xenopus embryo. Dev Biol. 1987;122:300–319. - PubMed
-
- Bauer DV, Huang S, Moody SA. The cleavage stage origin of Spemann’s organizer: analysis of the movements of blastomere clones before and during gastrulation in Xenopus. Development. 1994;120:1179–1189. - PubMed
-
- Saxén L. Organogenesis of the kidney. Cambridge University Press; Cambridge: 1987.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

