PDGF induces SphK1 expression via Egr-1 to promote pulmonary artery smooth muscle cell proliferation
- PMID: 27099350
- PMCID: PMC4935200
- DOI: 10.1152/ajpcell.00059.2016
PDGF induces SphK1 expression via Egr-1 to promote pulmonary artery smooth muscle cell proliferation
Abstract
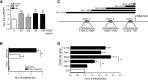
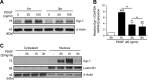
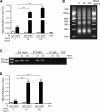
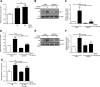
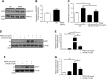
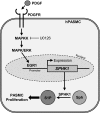
Pulmonary arterial hypertension (PAH) is a progressive, life-threatening disease for which there is currently no curative treatment available. Pathologic changes in this disease involve remodeling of the pulmonary vasculature, including marked proliferation of pulmonary artery smooth muscle cells (PASMCs). Recently, the bioactive lipid sphingosine-1-phosphate (S1P) and its activating kinase, sphingosine kinase 1 (SphK1), have been shown to be upregulated in PAH and promote PASMC proliferation. The mechanisms regulating the transcriptional upregulation of SphK1 in PASMCs are unknown. In this study, we investigated the role of platelet-derived growth factor (PDGF), a PAH-relevant stimuli associated with enhanced PASMC proliferation, on SphK1 expression regulation. In human PASMCs (hPASMCs), PDGF significantly increased SphK1 mRNA and protein expression and induced cell proliferation. Selective inhibition of SphK1 attenuated PDGF-induced hPASMC proliferation. In silico promoter analysis for SphK1 identified several binding sites for early growth response protein 1 (Egr-1), a PDGF-associated transcription factor. Luciferase assays demonstrated that PDGF activates the SphK1 promoter in hPASMCs, and truncation of the 5'-promoter reduced PDGF-induced SphK1 expression. Stimulation of hPASMCs with PDGF induced Egr-1 protein expression, and direct binding of Egr-1 to the SphK1 promoter was confirmed by chromatin immunoprecipitation analysis. Inhibition of ERK signaling prevented induction of Egr-1 by PDGF. Silencing of Egr-1 attenuated PDGF-induced SphK1 expression and hPASMC proliferation. These studies demonstrate that SphK1 is regulated by PDGF in hPASMCs via the transcription factor Egr-1, promoting cell proliferation. This novel mechanism of SphK1 regulation may be a therapeutic target in pulmonary vascular remodeling in PAH.
Keywords: Egr1; PASMC; PDGF; SphK1; gene expression; proliferation.
Copyright © 2016 the American Physiological Society.
Figures

References
-
- Agbani EO, Coats P, Mills A, Wadsworth RM. Peroxynitrite stimulates pulmonary artery endothelial and smooth muscle cell proliferation: involvement of ERK and PKC. Pulm Pharmacol Ther 24: 100–109, 2011. - PubMed
-
- Al Husseini A, Bagnato G, Farkas L, Gomez-Arroyo J, Farkas D, Mizuno S, Kraskauskas D, Abbate A, Van Tassel B, Voelkel NF, Bogaard HJ. Thyroid hormone is highly permissive in angioproliferative pulmonary hypertension in rats. Eur Respir J 41: 104–114, 2013. - PubMed
-
- Antoniu SA. Targeting PDGF pathway in pulmonary arterial hypertension. Expert Opin Ther Targets 16: 1055–1063, 2012. - PubMed
-
- Banks MF, Gerasimovskaya EV, Tucker DA, Frid MG, Carpenter TC, Stenmark KR. Egr-1 antisense oligonucleotides inhibit hypoxia-induced proliferation of pulmonary artery adventitial fibroblasts. J Appl Physiol (1985) 98: 732–738, 2005. - PubMed
-
- Berg JT, Breen EC, Fu Z, Mathieu-Costello O, West JB. Alveolar hypoxia increases gene expression of extracellular matrix proteins and platelet-derived growth factor-B in lung parenchyma. Am J Respir Crit Care Med 158: 1920–1928, 1998. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

