Transthyretin variants with improved inhibition of β-amyloid aggregation
- PMID: 27099354
- PMCID: PMC4867095
- DOI: 10.1093/protein/gzw008
Transthyretin variants with improved inhibition of β-amyloid aggregation
Abstract
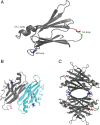
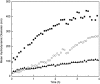
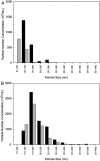
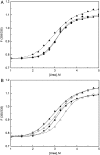
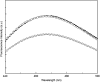
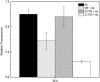
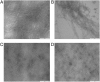
Aggregation of β-amyloid (Aβ) is widely believed to cause neuronal dysfunction in Alzheimer's disease. Transthyretin (TTR) binds to Aβ and inhibits its aggregation and neurotoxicity. TTR is a homotetrameric protein, with each monomer containing a short α-helix and two anti-parallel β-sheets. Dimers pack into tetramers to form a hydrophobic cavity. Here we report the discovery of a TTR mutant, N98A, that was more effective at inhibiting Aβ aggregation than wild-type (WT) TTR, although N98A and WT bound Aβ equally. The N98A mutation is located on a flexible loop distant from the putative Aβ-binding sites and does not alter secondary and tertiary structures nor prevent correct assembly into tetramers. Under non-physiological conditions, N98A tetramers were kinetically and thermodynamically less stable than WT, suggesting a difference in the tetramer folded structure. In vivo, the lone cysteine in TTR is frequently modified by S-cysteinylation or S-sulfonation. Like the N98A mutation, S-cysteinylation of TTR modestly decreased tetramer stability and increased TTR's effectiveness at inhibiting Aβ aggregation. Collectively, these data indicate that a subtle change in TTR tetramer structure measurably increases TTR's ability to inhibit Aβ aggregation.
Keywords: amyloid; protein aggregation; transthyretin.
© The Author 2016. Published by Oxford University Press. All rights reserved. For Permissions, please e-mail: journals.permissions@oup.com.
Figures



Similar articles
-
Copper mediated amyloid-β binding to Transthyretin.Sci Rep. 2018 Sep 13;8(1):13744. doi: 10.1038/s41598-018-31808-5. Sci Rep. 2018. PMID: 30213975 Free PMC article.
-
The inhibition of cellular toxicity of amyloid-β by dissociated transthyretin.J Biol Chem. 2020 Oct 9;295(41):14015-14024. doi: 10.1074/jbc.RA120.013440. Epub 2020 Aug 7. J Biol Chem. 2020. PMID: 32769117 Free PMC article.
-
Transthyretin as both a sensor and a scavenger of β-amyloid oligomers.Biochemistry. 2013 Apr 30;52(17):2849-61. doi: 10.1021/bi4001613. Epub 2013 Apr 19. Biochemistry. 2013. PMID: 23570378 Free PMC article.
-
The Positive Side of the Alzheimer's Disease Amyloid Cross-Interactions: The Case of the Aβ 1-42 Peptide with Tau, TTR, CysC, and ApoA1.Molecules. 2020 May 23;25(10):2439. doi: 10.3390/molecules25102439. Molecules. 2020. PMID: 32456156 Free PMC article. Review.
-
Transthyretin as a therapeutic target: the future of disease-modifying therapies for Alzheimer's disease.Mol Biol Rep. 2025 Apr 8;52(1):370. doi: 10.1007/s11033-025-10485-4. Mol Biol Rep. 2025. PMID: 40195175 Review.
Cited by
-
Endogenous Human Proteins Interfering with Amyloid Formation.Biomolecules. 2022 Mar 14;12(3):446. doi: 10.3390/biom12030446. Biomolecules. 2022. PMID: 35327638 Free PMC article. Review.
-
Cerebrospinal Fluid Proteins as Regulators of Beta-amyloid Aggregation and Toxicity.Isr J Chem. 2017 Jul;57(7-8):602-612. doi: 10.1002/ijch.201600078. Epub 2017 Jan 18. Isr J Chem. 2017. PMID: 29129937 Free PMC article.
-
Inhibition of curli assembly and Escherichia coli biofilm formation by the human systemic amyloid precursor transthyretin.Proc Natl Acad Sci U S A. 2017 Nov 14;114(46):12184-12189. doi: 10.1073/pnas.1708805114. Epub 2017 Oct 30. Proc Natl Acad Sci U S A. 2017. PMID: 29087319 Free PMC article.
-
Copper mediated amyloid-β binding to Transthyretin.Sci Rep. 2018 Sep 13;8(1):13744. doi: 10.1038/s41598-018-31808-5. Sci Rep. 2018. PMID: 30213975 Free PMC article.
-
Mechanisms of Transthyretin Inhibition of IAPP Amyloid Formation.Biomolecules. 2021 Mar 10;11(3):411. doi: 10.3390/biom11030411. Biomolecules. 2021. PMID: 33802170 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

