Minimal Residual Disease Evaluation in Childhood Acute Lymphoblastic Leukemia: A Clinical Evidence Review
- PMID: 27099643
- PMCID: PMC4808716
Minimal Residual Disease Evaluation in Childhood Acute Lymphoblastic Leukemia: A Clinical Evidence Review
Abstract
Background: Leukemia accounts for nearly a third of childhood cancers in Canada, with acute lymphoblastic leukemia (ALL) comprising nearly 80% of cases. Identification of prognostic factors that allow risk stratification and tailored treatment have improved overall survival. However, nearly a quarter of patients considered standard risk on the basis of conventional prognostic factors still relapse, and relapse is associated with increased morbidity and mortality. Relapse is thought to result from extremely low levels of leukemic cells left over once complete remission is reached, termed minimal residual disease (MRD). Poor event-free survival (EFS) as well as overall survival for those who are classified as MRD-positive have been substantiated in seminal studies demonstrating the prognostic value of MRD for EFS in the past few decades. This review sought to further elucidate the relationship between MRD and EFS by looking at relapse, the primary determinant of EFS and the biological mechanism through which MRD is thought to act. This evidence review aimed to ascertain whether MRD is an independent prognostic factor for relapse and to assess the effect of MRD-directed treatment on patient-important outcomes in childhood ALL.
Methods: Large prospective cohort studies with a priori multivariable analysis that includes potential confounders are required to draw confirmatory conclusions about the independence of a prognostic factor. Data on the prognostic value of MRD for relapse measured by molecular methods (polymerase chain reaction [PCR] of immunoglobulin or T-cell receptor rearrangements) or flow cytometry for leukemia-associated immunophenotypes or difference-from-normal approach were abstracted from included studies. Relevant data on relapse, EFS, and overall survival were abstracted from randomized controlled trials (RCTs) evaluating the effect of MRD-directed treatment.
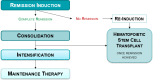
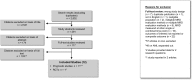
Results: A total of 2,832 citations were reviewed, of which 12 studies were included in this review. All cohort studies evaluating MRD as a prognostic factor for relapse found significant independent value when added to various existing prognostic factors. Seven studies showed prognostic value of MRD measured at the end of induction therapy and two at the end of consolidation therapy in de novo ALL, one study in relapsed ALL after re-induction therapy, and three studies before hematopoietic stem cell transplant. One large RCT in standard-risk patients found no compromise to outcomes when reducing treatment in MRD-negative patients, and also showed a 45% reduction in relapse risk and nearly 40% benefit in EFS when escalating treatment in MRD-positive patients.
Conclusions: Minimal residual disease is an independent prognostic factor for relapse in childhood ALL. Relapse is a key determinant of EFS and patients' quality of life. Treatment selected on the basis of MRD status appears to improve outcomes.
Figures



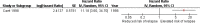



References
-
- Leukemia & Lymphoma Society of Canada. Leukemia [Internet]. 2013. [updated 10 July 2013]. Available from: http://www.llscanada.org/#/diseaseinformation/leukemia/
-
- Canadian Cancer Society's Advisory Committee on Cancer Statistics. Canadian Cancer Statistics 2014 [Internet]. Toronto, ON: Canadian Cancer Society; 2014. May [cited 2014 Nov 21]. Available from: http://www.cancer.ca/∼/media/cancer.ca/CW/cancer%20information/cancer%20...
-
- National Cancer Institute. PDQ® childhood acute lymphoblastic leukemia treatment [Internet]. Bethesda (MD): The Institute; 2014. Nov [cited 2014 Nov 21]. Available from: http://cancer.gov/cancertopics/pdq/treatment/childALL/Patient
-
- National Cancer Institute. PDQ Childhood Acute Myeloid Leukemia/Other Myeloid Malignancies Treatment [Internet]. Bethesda, MD: National Cancer Institute; 2014. Nov [cited 2014 Nov 21]. Available from: http://cancer.gov/cancertopics/pdq/treatment/childAML/Patient
-
- Macleod RA, Kaufmann M, Drexler HG. Cytogenetic analysis of cancer cell lines. Methods Mol Biol. 2011; 731: 57–78. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous
