Inducible T-cell co-stimulator ligand (ICOSL) blockade leads to selective inhibition of anti-KLH IgG responses in subjects with systemic lupus erythematosus
- PMID: 27099766
- PMCID: PMC4836284
- DOI: 10.1136/lupus-2016-000146
Inducible T-cell co-stimulator ligand (ICOSL) blockade leads to selective inhibition of anti-KLH IgG responses in subjects with systemic lupus erythematosus
Abstract
Objectives: To evaluate the safety, tolerability, pharmacokinetics (PK) and pharmacodynamics (PD) of single-dose and multiple-dose administration of AMG 557, a human anti-inducible T cell co-stimulator ligand (ICOSL) monoclonal antibody, in subjects with systemic lupus erythematosus (SLE).
Methods: Patients with mild, stable SLE (n=112) were enrolled in two clinical trials to evaluate the effects of single (1.8-210 mg subcutaneous or 18 mg intravenous) and multiple (6 -210 mg subcutaneous every other week (Q2W)×7) doses of AMG 557. Subjects received two 1 mg intradermal injections 28 days apart of keyhole limpet haemocyanin (KLH), a neoantigen, to assess PD effects of AMG 557. Safety, PK, target occupancy, anti-KLH antibody responses, lymphocyte subset analyses and SLE-associated biomarkers and clinical outcomes were assessed.
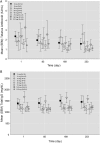
Results: AMG 557 demonstrated an acceptable safety profile. The PK properties were consistent with an antibody directed against a cell surface target, with non-linear PK observed at lower concentrations and linear PK at higher concentrations. Target occupancy by AMG 557 was dose dependent and reversible, and maximal occupancy was achieved in the setting of this trial. Anti-AMG 557 antibodies were observed, but none were neutralising and without impact on drug levels. A significant reduction in the anti-KLH IgG response was observed with AMG 557 administration without discernible changes in the anti-KLH IgM response or on the overall IgG levels. No discernible changes were seen in lymphocyte subsets or in SLE-related biomarkers and clinical measures.
Conclusions: The selective reduction in anti-KLH IgG demonstrates a PD effect of AMG 557 in subjects with SLE consistent with the biology of the ICOS pathway and supports further studies of AMG 557 as a potential therapeutic for autoimmune diseases.
Trial registration numbers: NCT02391259 and NCT00774943.
Keywords: B cells; Inflammation; Systemic Lupus Erythematosus; T Cells.
Figures





References
-
- Nurieva RI, Liu X, Dong C. Yin-Yang of costimulation: crucial controls of immune tolerance and function. Immunol Rev 2009;229:88–100. doi:10.1111/j.1600-065X.2009.00769.x - DOI - PMC - PubMed
-
- Ford ML, Adams AB, Pearson TC. Targeting co-stimulatory pathways: transplantation and autoimmunity. Nat Rev Nephrol 2014;10:14–24. doi:10.1038/nrneph.2013.183 - DOI - PMC - PubMed
-
- Zitvogel L, Kroemer G. Targeting PD-1/PD-L1 interactions for cancer immunotherapy. Oncoimmunology 2012;1:1223–5. doi:10.4161/onci.21335 - DOI - PMC - PubMed
-
- Chen L, Flies DB. Molecular mechanisms of T cell co-stimulation and co-inhibition. Nat Rev Immunol 2013;13:227–42. nri3405http://dx.doi.org/10.1038/nri3405 - DOI - PMC - PubMed
-
- Choi YS, Kageyama R, Eto D et al. . ICOS receptor instructs T follicular helper cell versus effector cell differentiation via induction of the transcriptional repressor Bcl6. Immunity 2011;34:932–46. doi:10.1016/j.immuni.2011.03.023 - DOI - PMC - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
