MicroRNAs: The Role in Autoimmune Inflammation
- PMID: 27099782
- PMCID: PMC4837569
MicroRNAs: The Role in Autoimmune Inflammation
Abstract
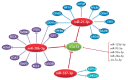
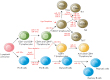
MicroRNAs (miRNAs) are small non-coding RNA molecules that regulate gene expression at the post-transcriptional level through base-pairing predominantly with a 3'-untranslated region of target mRNA, followed by mRNA degradation or translational repression. Totally, miRNAs change, through a complex regulatory network, the expression of more than 60% of human genes. MiRNAs are key regulators of the immune response that affect maturation, proliferation, differentiation, and activation of immune cells, as well as antibody secretion and release of inflammatory mediators. Disruption of this regulation may lead to the development of various pathological conditions, including autoimmune inflammation. This review summarizes the data on biogenesis and the mechanisms of miRNA action. We discuss the role of miRNAs in the development and the action of the immune system, as well as in the development of an autoimmune inflammatory response. Special attention is given to the role of miRNAs in the autoimmune inflammation in multiple sclerosis, which is a serious socially significant disease of the central nervous system. Currently, a lot of research is focused on this problem.
Keywords: autoimmune inflammation; microRNA; multiple sclerosis.
Figures


References
LinkOut - more resources
Full Text Sources
