Pharmacokinetic analysis and comparison of caffeine administered rapidly or slowly in coffee chilled or hot versus chilled energy drink in healthy young adults
- PMID: 27100333
- PMCID: PMC4898153
- DOI: 10.3109/15563650.2016.1146740
Pharmacokinetic analysis and comparison of caffeine administered rapidly or slowly in coffee chilled or hot versus chilled energy drink in healthy young adults
Abstract
Context: There is a paucity of data describing the impact of type of beverage (coffee versus energy drink), different rates of consumption and different temperature of beverages on the pharmacokinetic disposition of caffeine. Additionally, there is concern that inordinately high levels of caffeine may result from the rapid consumption of cold energy drinks.
Objective: The objective of this study was to compare the pharmacokinetics of caffeine under various drink temperature, rate of consumption and vehicle (coffee versus energy drink) conditions.
Materials: Five caffeine (dose = 160 mg) conditions were evaluated in an open-label, group-randomized, crossover fashion. After the administration of each caffeine dose, 10 serial plasma samples were harvested. Caffeine concentration was measured via liquid chromatography-mass spectrometry (LC-MS), and those concentrations were assessed by non-compartmental pharmacokinetic analysis. The calculated mean pharmacokinetic parameters were analyzed statistically by one-way repeated measures analysis of variance (RM ANOVA). If differences were found, each group was compared to the other by all pair-wise multiple comparison.
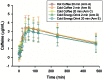
Results: Twenty-four healthy subjects ranging in age from 18 to 30 completed the study. The mean caffeine concentration time profiles were similar with overlapping SDs at all measured time points. The ANOVA revealed significant differences in mean Cmax and Vd ss/F, but no pair-wise comparisons reached statistical significance. No other differences in pharmacokinetic parameters were found.
Discussion: The results of this study are consistent with previous caffeine pharmacokinetic studies and suggest that while rate of consumption, temperature of beverage and vehicle (coffee versus energy drink) may be associated with slightly different pharmacokinetic parameters, the overall impact of these variables is small.
Conclusion: This study suggests that caffeine absorption and exposure from coffee and energy drink is similar irrespective of beverage temperature or rate of consumption.
Keywords: Caffeine; coffee; energy drinks; pharmacokinetics; rapid administration.
Figures
References
-
- Higdon J, Frei B. Coffee and Health: a review of recent human research. Crit Rev Food Sci Nutr. 2006;46:101–123. - PubMed
-
- FDA Safety Alerts & Advisories . FDA consumer advice on pure powdered caffeine. 2015. http://www.fda.gov/Food/RecallsOutbreaksEmergencies/SafetyAlertsAdvisori...
-
- Simon M, Mosher J. Eat drink politics. 2007. http://www.eatdrinkpolitics.com/wp-content/uploads/AEDReportSimon2007.pdf
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical