The combination of HLA-B*15:01 and DRB1*15:01 is associated with gemcitabine plus erlotinib-induced interstitial lung disease in patients with advanced pancreatic cancer
- PMID: 27100735
- PMCID: PMC4882349
- DOI: 10.1007/s00280-016-3026-6
The combination of HLA-B*15:01 and DRB1*15:01 is associated with gemcitabine plus erlotinib-induced interstitial lung disease in patients with advanced pancreatic cancer
Abstract
Purpose: In a phase III study of gemcitabine plus erlotinib for advanced pancreatic cancer conducted in Canada, the incidence of interstitial lung disease (ILD) was 3.5 %. However, the incidence of ILD was reported as high as 8.5 % in a Japanese phase II study. These results suggest the influence of ethnic factors in the association of the use of gemcitabine plus erlotinib with the incidence of ILD. Here, we conducted a prospective study to analyze the relationship between human leukocyte antigen (HLA) alleles and ILD in Japanese patients with advanced pancreatic cancer receiving gemcitabine plus erlotinib.
Methods: Patients were treated with gemcitabine (1000 mg/m(2); administered by intravenous infusion on days 1, 8, and 15 every 4 weeks) and erlotinib (given orally at 100 mg/day). We compared the frequencies of HLA alleles in patients who did and did not develop ILD.
Results: A total of 57 patients were treated, and 4 patients (7.0 %) developed ILD. The combination of HLA-B*15:01 and DRB1*15:01 was observed in 2 of 4 patients (50 %) with ILD and in only 1 of 53 patients without ILD (2 %) resulting in odds ratio of 52.0 (95 % CI 3.2-842.5; p = 0.011).
Conclusion: These results suggest that the combination of HLA-B*15:01 and DRB1*15:01 is associated with ILD in Japanese patients with advanced pancreatic cancer receiving gemcitabine plus erlotinib.
Keywords: Erlotinib; Gemcitabine; Human leukocyte antigen; Interstitial lung disease; Pancreatic cancer.
Figures
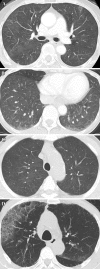
References
-
- Moore MJ, Goldstein D, Hamm J, Figer A, Hecht JR, Gallinger S, Au HJ, Murawa P, Walde D, Wolff RA, Campos D, Lim R, Ding K, Clark G, Voskoglou-Nomikos T, Ptasynski M, Parulekar W, National Cancer Institute of Canada Clinical Trials G Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2007;25(15):1960–1966. doi: 10.1200/JCO.2006.07.9525. - DOI - PubMed
-
- Gemma A, Kudoh S, Ando M, Ohe Y, Nakagawa K, Johkoh T, Yamazaki N, Arakawa H, Inoue Y, Ebina M, Kusumoto M, Kuwano K, Sakai F, Taniguchi H, Fukuda Y, Seki A, Ishii T, Fukuoka M. Final safety and efficacy of erlotinib in the phase 4 POLARSTAR surveillance study of 10 708 Japanese patients with non-small-cell lung cancer. Cancer Sci. 2014;105(12):1584–1590. doi: 10.1111/cas.12550. - DOI - PMC - PubMed
-
- Shepherd FA, Rodrigues Pereira J, Ciuleanu T, Tan EH, Hirsh V, Thongprasert S, Campos D, Maoleekoonpiroj S, Smylie M, Martins R, van Kooten M, Dediu M, Findlay B, Tu D, Johnston D, Bezjak A, Clark G, Santabarbara P, Seymour L, National Cancer Institute of Canada Clinical Trials G Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med. 2005;353(2):123–132. doi: 10.1056/NEJMoa050753. - DOI - PubMed
-
- Okusaka T, Furuse J, Funakoshi A, Ioka T, Yamao K, Ohkawa S, Boku N, Komatsu Y, Nakamori S, Iguchi H, Ito T, Nakagawa K, Nakachi K. Phase II study of erlotinib plus gemcitabine in Japanese patients with unresectable pancreatic cancer. Cancer Sci. 2011;102(2):425–431. doi: 10.1111/j.1349-7006.2010.01810.x. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

