Gfap-positive radial glial cells are an essential progenitor population for later-born neurons and glia in the zebrafish spinal cord
- PMID: 27100776
- PMCID: PMC4918407
- DOI: 10.1002/glia.22990
Gfap-positive radial glial cells are an essential progenitor population for later-born neurons and glia in the zebrafish spinal cord
Abstract
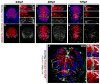
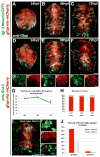
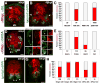
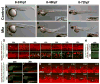
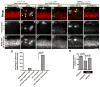
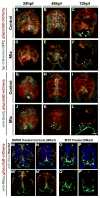
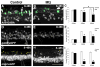
Radial glial cells are presumptive neural stem cells (NSCs) in the developing nervous system. The direct requirement of radial glia for the generation of a diverse array of neuronal and glial subtypes, however, has not been tested. We employed two novel transgenic zebrafish lines and endogenous markers of NSCs and radial glia to show for the first time that radial glia are essential for neurogenesis during development. By using the gfap promoter to drive expression of nuclear localized mCherry we discerned two distinct radial glial-derived cell types: a major nestin+/Sox2+ subtype with strong gfap promoter activity and a minor Sox2+ subtype lacking this activity. Fate mapping studies in this line indicate that gfap+ radial glia generate later-born CoSA interneurons, secondary motorneurons, and oligodendroglia. In another transgenic line using the gfap promoter-driven expression of the nitroreductase enzyme, we induced cell autonomous ablation of gfap+ radial glia and observed a reduction in their specific derived lineages, but not Blbp+ and Sox2+/gfap-negative NSCs, which were retained and expanded at later larval stages. Moreover, we provide evidence supporting classical roles of radial glial in axon patterning, blood-brain barrier formation, and locomotion. Our results suggest that gfap+ radial glia represent the major NSC during late neurogenesis for specific lineages, and possess diverse roles to sustain the structure and function of the spinal cord. These new tools will both corroborate the predicted roles of astroglia and reveal novel roles related to development, physiology, and regeneration in the vertebrate nervous system. GLIA 2016;64:1170-1189.
Keywords: genetic ablation; neural stem cell; neurogenesis; radial glia; spinal cord; zebrafish.
© 2016 Wiley Periodicals, Inc.
Conflict of interest statement
Conflict of interest: Nothing to report.
Figures

References
-
- Anthony TE, Klein C, Fishell G, Heintz N. Radial glia serve as neuronal progenitors in all regions of the central nervous system. Neuron. 2004;41:881–890. - PubMed
-
- Appel B, Korzh V, Glasgow E, Thor S, Edlund T, Dawid IB, Eisen JS. Motoneuron fate specification revealed by patterned LIM homeobox gene expression in embryonic zebrafish. Development. 1995;121:4117–4125. - PubMed
-
- Barresi MJ, Hutson LD, Chien CB, Karlstrom RO. Hedgehog regulated Slit expression determines commissure and glial cell position in the zebrafish forebrain. Development. 2005;132:3643–3656. - PubMed
-
- Barry D, McDermott K. Differentiation of radial glia from radial precursor cells and transformation into astrocytes in the developing rat spinal cord. Glia. 2005;50:187–197. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

