CIP2A Promotes T-Cell Activation and Immune Response to Listeria monocytogenes Infection
- PMID: 27100879
- PMCID: PMC4839633
- DOI: 10.1371/journal.pone.0152996
CIP2A Promotes T-Cell Activation and Immune Response to Listeria monocytogenes Infection
Abstract
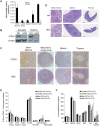
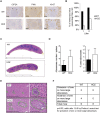
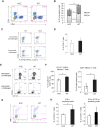
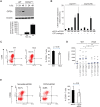
The oncoprotein Cancerous Inhibitor of Protein Phosphatase 2A (CIP2A) is overexpressed in most malignancies and is an obvious candidate target protein for future cancer therapies. However, the physiological importance of CIP2A-mediated PP2A inhibition is largely unknown. As PP2A regulates immune responses, we investigated the role of CIP2A in normal immune system development and during immune response in vivo. We show that CIP2A-deficient mice (CIP2AHOZ) present a normal immune system development and function in unchallenged conditions. However when challenged with Listeria monocytogenes, CIP2AHOZ mice display an impaired adaptive immune response that is combined with decreased frequency of both CD4+ T-cells and CD8+ effector T-cells. Importantly, the cell autonomous effect of CIP2A deficiency for T-cell activation was confirmed. Induction of CIP2A expression during T-cell activation was dependent on Zap70 activity. Thus, we reveal CIP2A as a hitherto unrecognized mediator of T-cell activation during adaptive immune response. These results also reveal CIP2AHOZ as a possible novel mouse model for studying the role of PP2A activity in immune regulation. On the other hand, the results also indicate that CIP2A targeting cancer therapies would not cause serious immunological side-effects.
Conflict of interest statement
Figures
References
-
- Homsi J, Walsh D, Panta R, Lagman R, Nelson KA, Longworth DL. Infectious complications of advanced cancer. Supportive Care in Cancer. 8(6):487–92. - PubMed
-
- Stosor V, Zembower TR, editors. Infectious Complications in Cancer Patients. Cham: Springer International Publishing; 2014.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

