The upcoming 3D-printing revolution in microfluidics
- PMID: 27101171
- PMCID: PMC4862901
- DOI: 10.1039/c6lc00163g
The upcoming 3D-printing revolution in microfluidics
Abstract
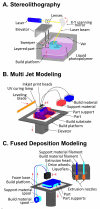
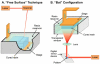
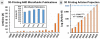
In the last two decades, the vast majority of microfluidic systems have been built in poly(dimethylsiloxane) (PDMS) by soft lithography, a technique based on PDMS micromolding. A long list of key PDMS properties have contributed to the success of soft lithography: PDMS is biocompatible, elastomeric, transparent, gas-permeable, water-impermeable, fairly inexpensive, copyright-free, and rapidly prototyped with high precision using simple procedures. However, the fabrication process typically involves substantial human labor, which tends to make PDMS devices difficult to disseminate outside of research labs, and the layered molding limits the 3D complexity of the devices that can be produced. 3D-printing has recently attracted attention as a way to fabricate microfluidic systems due to its automated, assembly-free 3D fabrication, rapidly decreasing costs, and fast-improving resolution and throughput. Resins with properties approaching those of PDMS are being developed. Here we review past and recent efforts in 3D-printing of microfluidic systems. We compare the salient features of PDMS molding with those of 3D-printing and we give an overview of the critical barriers that have prevented the adoption of 3D-printing by microfluidic developers, namely resolution, throughput, and resin biocompatibility. We also evaluate the various forces that are persuading researchers to abandon PDMS molding in favor of 3D-printing in growing numbers.
Figures













References
-
- Becker H, Locascio LE. Talanta. 2002;56:267–287. - PubMed
-
- Sackmann EK, Fulton AL, Beebe DJ. Nature. 2014;507:181–189. - PubMed
-
- Shankar SR, Jansson DG. In: Concurrent Engineering: Contemporary Issues and Modern Design Tools. Parsaei HR, Sullivan WG, editors. Springer; US: 1993.
-
- Roy E, Galas J-C, Veres T. Lab on a chip. 2011;11:3193–3196. - PubMed
-
- Focke M, Kosse D, Müller C, Reinecke H, Zengerle R, von Stetten F. Lab on a chip. 2010;10:1365–1386. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

