Glypican-1 nanoliposomes for potentiating growth factor activity in therapeutic angiogenesis
- PMID: 27101205
- PMCID: PMC5600893
- DOI: 10.1016/j.biomaterials.2016.03.048
Glypican-1 nanoliposomes for potentiating growth factor activity in therapeutic angiogenesis
Abstract
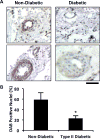
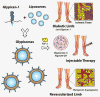
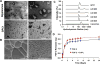
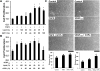
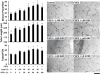
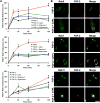
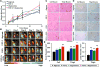
Therapeutic angiogenesis is a highly appealing concept for treating tissues that become ischemic due to vascular disease. A major barrier to the clinical translation of angiogenic therapies is that the patients that are in the greatest need of these treatments often have long term disease states and co-morbidities, such as diabetes and obesity, that make them resistant to angiogenic stimuli. In this study, we identified that human patients with type 2 diabetes have reduced levels of glypican-1 in the blood vessels of their skin. The lack of this key co-receptor in the tissue may make the application of exogenous angiogenic growth factors or cell therapies ineffective. We created a novel therapeutic enhancer for growth factor activity consisting of glypican-1 delivered in a nanoliposomal carrier (a "glypisome"). Here, we demonstrate that glypisomes enhance FGF-2 mediated endothelial cell proliferation, migration and tube formation. In addition, glypisomes enhance FGF-2 trafficking by increasing both uptake and endosomal processing. We encapsulated FGF-2 or FGF-2 with glypisomes in alginate beads and used these to deliver localized growth factor therapy in a murine hind limb ischemia model. Co-delivery of glypisomes with FGF-2 markedly increased the recovery of perfusion and vessel formation in ischemic hind limbs of wild type and diabetic mice in comparison to mice treated with FGF-2 alone. Together, our findings support that glypisomes are effective means for enhancing growth factor activity and may improve the response to local angiogenic growth factor therapies for ischemia.
Keywords: Angiogenesis; Fibroblast growth factor-2 (FGF-2); Glypican-1; Ischemia; Neovascularization; Peripheral arterial disease; Proteoliposomes; Vascular endothelial growth factor (VEGF).
Copyright © 2016 Elsevier Ltd. All rights reserved.
Figures

References
-
- Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FG. Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II) Journal of vascular surgery. 2007;(45 Suppl S):S5–67. - PubMed
-
- Udelson JE, Dilsizian V, Laham RJ, Chronos N, Vansant J, Blais M, et al. Therapeutic angiogenesis with recombinant fibroblast growth factor-2 improves stress and rest myocardial perfusion abnormalities in patients with severe symptomatic chronic coronary artery disease. Circulation. 2000;102:1605–10. - PubMed
-
- Lederman RJ, Mendelsohn FO, Anderson RD, Saucedo JF, Tenaglia AN, Hermiller JB, et al. Therapeutic angiogenesis with recombinant fibroblast growth factor-2 for intermittent claudication (the TRAFFIC study): a randomised trial. Lancet. 2002;359:2053–8. - PubMed
-
- Lederman RJ, Tenaglia AN, Anderson RD, Hermiller JB, Rocha-Singh K, Mendelsohn FO, et al. Design of the therapeutic angiogenesis with recombinant fibroblast growth factor-2 for intermittent claudication (TRAFFIC) trial. The American journal of cardiology. 2001;88:192–5. A6–7. - PubMed
-
- Rajagopalan S, Mohler ER, 3rd, Lederman RJ, Mendelsohn FO, Saucedo JF, Goldman CK, et al. Regional angiogenesis with vascular endothelial growth factor in peripheral arterial disease: a phase II randomized, double-blind, controlled study of adenoviral delivery of vascular endothelial growth factor 121 in patients with disabling intermittent claudication. Circulation. 2003;108:1933–8. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

