Extracellular interactions and ligand degradation shape the nodal morphogen gradient
- PMID: 27101364
- PMCID: PMC4887204
- DOI: 10.7554/eLife.13879
Extracellular interactions and ligand degradation shape the nodal morphogen gradient
Abstract
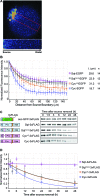
The correct distribution and activity of secreted signaling proteins called morphogens is required for many developmental processes. Nodal morphogens play critical roles in embryonic axis formation in many organisms. Models proposed to generate the Nodal gradient include diffusivity, ligand processing, and a temporal activation window. But how the Nodal morphogen gradient forms in vivo remains unclear. Here, we have measured in vivo for the first time, the binding affinity of Nodal ligands to their major cell surface receptor, Acvr2b, and to the Nodal inhibitor, Lefty, by fluorescence cross-correlation spectroscopy. We examined the diffusion coefficient of Nodal ligands and Lefty inhibitors in live zebrafish embryos by fluorescence correlation spectroscopy. We also investigated the contribution of ligand degradation to the Nodal gradient. We show that ligand clearance via degradation shapes the Nodal gradient and correlates with its signaling range. By computational simulations of gradient formation, we demonstrate that diffusivity, extra-cellular interactions, and selective ligand destruction collectively shape the Nodal morphogen gradient.
Keywords: Nodal; developmental biology; gradient; inhibitor; morphogen; receptor; stem cells; zebrafish.
Conflict of interest statement
The authors declare that no competing interests exist.
Figures






References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

