Subcortical connections of the perirhinal, postrhinal, and entorhinal cortices of the rat. II. efferents
- PMID: 27101786
- PMCID: PMC5070461
- DOI: 10.1002/hipo.22600
Subcortical connections of the perirhinal, postrhinal, and entorhinal cortices of the rat. II. efferents
Abstract
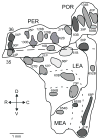
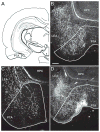
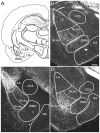
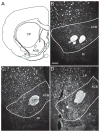
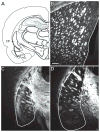
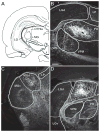
This is the second of two studies detailing the subcortical connections of the perirhinal (PER), the postrhinal (POR) and entorhinal (EC) cortices of the rat. In the present study, we analyzed the subcortical efferents of the rat PER areas 35 and 36, POR, and the lateral and medial entorhinal areas (LEA and MEA). Anterograde tracers were injected into these five regions, and the resulting density of fiber labeling was quantified in an extensive set of subcortical structures. Density and topography of fiber labeling were quantitatively assessed in 36 subcortical areas, including olfactory structures, claustrum, amygdala nuclei, septal nuclei, basal ganglia, thalamic nuclei, and hypothalamic structures. In addition to reporting the density of labeled fibers, we incorporated a new method for quantifying the size of anterograde projections that takes into account the volume of the target subcortical structure as well as the density of fiber labeling. The PER, POR, and EC displayed unique patterns of projections to subcortical areas. Interestingly, all regions examined provided strong input to the basal ganglia, although the projections arising in the PER and LEA were stronger and more widespread. PER areas 35 and 36 exhibited similar pattern of projections with some differences. PER area 36 projects more heavily to the lateral amygdala and much more heavily to thalamic nuclei including the lateral posterior nucleus, the posterior complex, and the nucleus reuniens. Area 35 projects more heavily to olfactory structures. The LEA provides the strongest and most widespread projections to subcortical structures including all those targeted by the PER as well as the medial and posterior septal nuclei. POR shows fewer subcortical projections overall, but contributes substantial input to the lateral posterior nucleus of the thalamus. The MEA projections are even weaker. Our results suggest that the PER and LEA have greater influence over olfactory, amygdala, and septal nuclei, whereas PER area 36 and the POR have greater influence over thalamic nuclei. © 2016 Wiley Periodicals, Inc.
Keywords: anatomy; anterograde; connectivity; memory; parahippocampal.
© 2016 Wiley Periodicals, Inc.
Figures

References
-
- Blackstad TW. Commissural connections of the hippocampal region in the rat, with special reference to their mode of termination. J Comp Neurol. 1956;105:417–537. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

