Systematic Analysis of Adverse Event Reports for Sex Differences in Adverse Drug Events
- PMID: 27102014
- PMCID: PMC4840306
- DOI: 10.1038/srep24955
Systematic Analysis of Adverse Event Reports for Sex Differences in Adverse Drug Events
Abstract
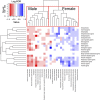
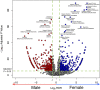
Increasing evidence has shown that sex differences exist in Adverse Drug Events (ADEs). Identifying those sex differences in ADEs could reduce the experience of ADEs for patients and could be conducive to the development of personalized medicine. In this study, we analyzed a normalized US Food and Drug Administration Adverse Event Reporting System (FAERS). Chi-squared test was conducted to discover which treatment regimens or drugs had sex differences in adverse events. Moreover, reporting odds ratio (ROR) and P value were calculated to quantify the signals of sex differences for specific drug-event combinations. Logistic regression was applied to remove the confounding effect from the baseline sex difference of the events. We detected among 668 drugs of the most frequent 20 treatment regimens in the United States, 307 drugs have sex differences in ADEs. In addition, we identified 736 unique drug-event combinations with significant sex differences. After removing the confounding effect from the baseline sex difference of the events, there are 266 combinations remained. Drug labels or previous studies verified some of them while others warrant further investigation.
Figures
References
-
- Lazarou J., Pomeranz B. H. & Corey P. N. Incidence of adverse drug reactions in hospitalized patients: a meta-analysis of prospective studies. Jama-J Am Med Assoc 279, 1200–1205 (1998). - PubMed
-
- Classen D. C., Pestotnik S. L., Evans R. S., Lloyd J. F. & Burke J. P. Adverse drug events in hospitalized patients-Excess length of stay, extra costs, and attributable mortality. Jama-J Am Med Assoc 277, 301–306 (1997). - PubMed
-
- Hartung T. Toxicology for the twenty-first century. Nature 460, 208–212 (2009). - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

