Conditional deletion of Sox17 reveals complex effects on uterine adenogenesis and function
- PMID: 27102016
- PMCID: PMC5521196
- DOI: 10.1016/j.ydbio.2016.04.010
Conditional deletion of Sox17 reveals complex effects on uterine adenogenesis and function
Abstract
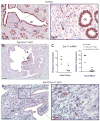
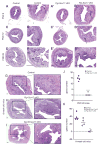
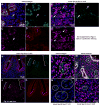
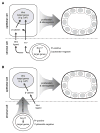
The importance of canonical Wnt signaling to murine uterine development is well established. Mouse models in which uterine-specific Wnt ligands, β-catenin, or Lef1 are disrupted result in failure of postnatal endometrial gland development. Sox17 is a transcription factor characterized in numerous tissues as an antagonist of Wnt signaling. Thus, we hypothesized that conditional ablation of Sox17 would lead to hyperproliferation of endometrial glands in mice. Contrary to our prediction, disruption of Sox17 in epithelial and stromal compartments led to inhibition of endometrial adenogenesis and a loss of reproductive capacity. Epithelium-specific Sox17 disruption resulted in normal adenogenesis although reproductive capacity remained impaired. These findings suggest that non-epithelial, Sox17-positive cells are necessary for adenogenesis and that glands require Sox17 to properly function. To our knowledge, these findings are the first to implicate Sox17 in endometrial gland formation and reproductive success. The data presented herein underscore the importance of studying Sox17 in uterine homeostasis and function.
Keywords: Adenogenesis; Development; Endometrial glands; Mouse model; Sox17; Uterus.
Copyright © 2016 Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors have no competing financial interests.
Figures

References
-
- Brody JR, Cunha GR. Histologic, morphometric, and immunocytochemical analysis of myometrial development in rats and mice: I. Normal development. Am J Anat. 1989;186:1–20. - PubMed
-
- Contreras CM, Akbay EA, Gallardo TD, Haynie JM, Sharma S, Tagao O, Bardeesy N, Takahashi M, Settleman J, Wong KK, et al. Lkb1 inactivation is sufficient to drive endometrial cancers that are aggressive yet highly responsive to mTOR inhibitor monotherapy. Disease models & mechanisms. 2010;3:181–193. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

