Bone marrow-derived mesenchymal stem cells improve diabetes-induced cognitive impairment by exosome transfer into damaged neurons and astrocytes
- PMID: 27102354
- PMCID: PMC4840335
- DOI: 10.1038/srep24805
Bone marrow-derived mesenchymal stem cells improve diabetes-induced cognitive impairment by exosome transfer into damaged neurons and astrocytes
Abstract
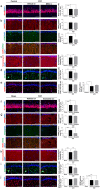
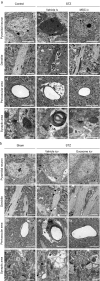
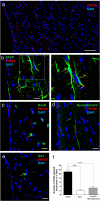
The incidence of dementia is higher in diabetic patients, but no effective treatment has been developed. This study showed that rat bone marrow mesenchymal stem cells (BM-MSCs) can improve the cognitive impairments of STZ-diabetic mice by repairing damaged neurons and astrocytes. The Morris water maze test demonstrated that cognitive impairments induced by diabetes were significantly improved by intravenous injection of BM-MSCs. In the CA1 region of the hippocampus, degeneration of neurons and astrocytes, as well as synaptic loss, were prominent in diabetes, and BM-MSC treatment successfully normalized them. Since a limited number of donor BM-MSCs was observed in the brain parenchyma, we hypothesized that humoral factors, especially exosomes released from BM-MSCs, act on damaged neurons and astrocytes. To investigate the effectiveness of exosomes for treatment of diabetes-induced cognitive impairment, exosomes were purified from the culture media and injected intracerebroventricularly into diabetic mice. Recovery of cognitive impairment and histological abnormalities similar to that seen with BM-MSC injection was found following exosome treatment. Use of fluorescence-labeled exosomes demonstrated that injected exosomes were internalized into astrocytes and neurons; these subsequently reversed the dysfunction. The present results indicate that exosomes derived from BM-MSCs might be a promising therapeutic tool for diabetes-induced cognitive impairment.
Figures


References
-
- Leibson C. L. et al.. Risk of dementia among persons with diabetes mellitus: a population-based cohort study. Am J Epidemiol 145, 301–308 (1997). - PubMed
-
- Ott A. et al.. Diabetes mellitus and the risk of dementia: The Rotterdam Study. Neurology 53, 1937–1942 (1999). - PubMed
-
- Sakata A. et al.. Improvement of cognitive impairment in female type 2 diabetes mellitus mice by spironolactone. J Renin Angiotensin Aldosterone Syst 13, 84–90 (2012). - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

