Ruxolitinib versus best available therapy in patients with polycythemia vera: 80-week follow-up from the RESPONSE trial
- PMID: 27102499
- PMCID: PMC5004461
- DOI: 10.3324/haematol.2016.143644
Ruxolitinib versus best available therapy in patients with polycythemia vera: 80-week follow-up from the RESPONSE trial
Abstract
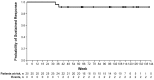
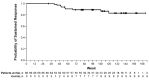
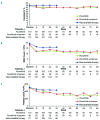
RESPONSE is an open-label phase 3 study evaluating the Janus kinase 1/Janus kinase 2 inhibitor ruxolitinib versus best available therapy for efficacy/safety in hydroxyurea-resistant or intolerant patients with polycythemia vera. This preplanned analysis occurred when all patients completed the Week 80 visit or discontinued. Objectives included evaluating the durability of the primary response (Week 32 phlebotomy-independent hematocrit control plus ≥35% spleen volume reduction), its components, and that of complete hematologic remission; and long-term safety. Median exposure was 111 weeks; 91/110 (82.7%) patients randomized to ruxolitinib remained on treatment. No patients continued best available therapy (98/112 [87.5%] crossed over to ruxolitinib, most at/soon after Week 32). At Week 32, primary response was achieved by 22.7% vs. 0.9% of patients randomized to ruxolitinib and best available therapy, respectively (hematocrit control, 60.0% vs. 18.8%; spleen response, 40.0% vs. 0.9%). The probability of maintaining primary and hematocrit responses for ≥80 weeks was 92% and 89%, respectively; 43/44 spleen responses were maintained until Week 80. Complete hematologic remission at Week 32 was achieved in 23.6% of ruxolitinib-randomized patients; the probability of maintaining complete hematologic remission for ≥80 weeks was 69%. Among ruxolitinib crossover patients, 79.2% were not phlebotomized, and 18.8% achieved a ≥35% reduction from baseline in spleen volume after 32 weeks of treatment. New or worsening hematologic laboratory abnormalities in ruxolitinib-treated patients were primarily grade 1/2 decreases in hemoglobin, lymphocytes, and platelets. The thromboembolic event rate per 100 patient-years was 1.8 with randomized ruxolitinib treatment vs. 8.2 with best available therapy. These data support ruxolitinib as an effective long-term treatment option for hydroxyurea-resistant or intolerant patients with polycythemia vera. This trial was registered at clinicaltrials.gov identifier: 01243944.
Trial registration: ClinicalTrials.gov NCT01243944.
Copyright© Ferrata Storti Foundation.
Figures
References
-
- Tefferi A, Vardiman JW. Classification and diagnosis of myeloproliferative neoplasms: the 2008 World Health Organization criteria and point-of-care diagnostic algorithms. Leukemia. 2008;22(1):14–22. - PubMed
-
- Stein BL, Moliterno AR, Tiu RV. Polycythemia vera disease burden: contributing factors, impact on quality of life, and emerging treatment options. Ann Hematol. 2014;93(12):1965–1976. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

